Qing-dai powder promotes recovery of colitis by inhibiting inflammatory responses of colonic macrophages in dextran sulfate sodium-treated mice
- PMID: 26464580
- PMCID: PMC4604072
- DOI: 10.1186/s13020-015-0061-x
Qing-dai powder promotes recovery of colitis by inhibiting inflammatory responses of colonic macrophages in dextran sulfate sodium-treated mice
Abstract
Background: Qing-dai powder (QDP), comprising Indigo naturalis (Qing-dai) and dried alum (Ku-fan), was used in Chinese medicine to treat the conditions associated with mucosal hemorrhage, such as ulcerative colitis (UC). This study aims to investigate the effects and potential mechanism of QDP on dextran sulfate sodium (DSS)-induced acute colitis in mice and to examine the regulatory effects of QDP on macrophages.
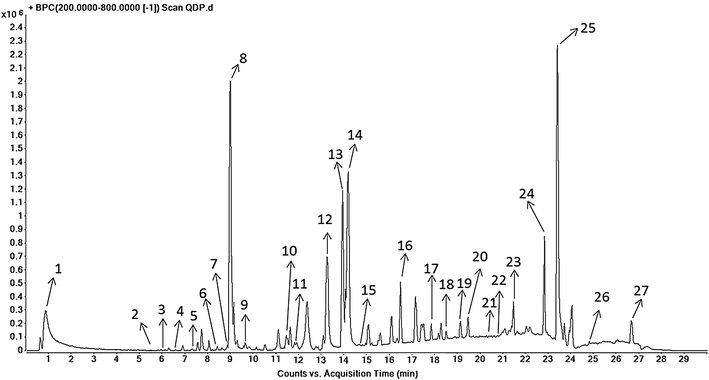
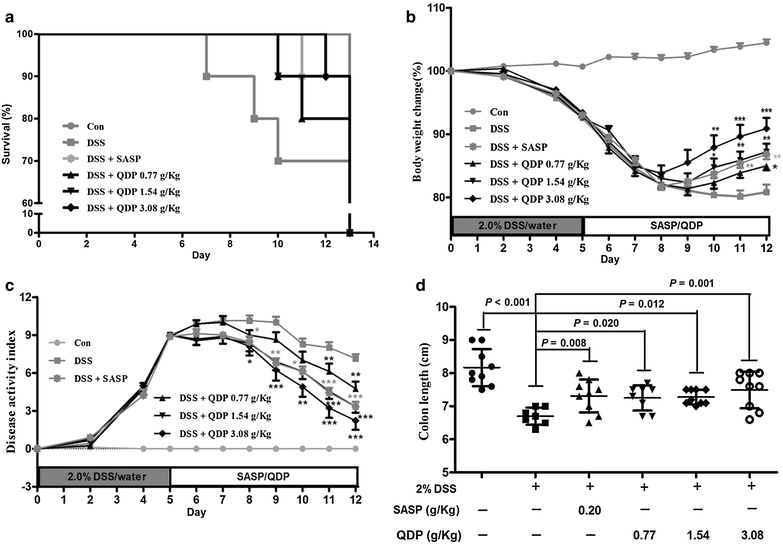
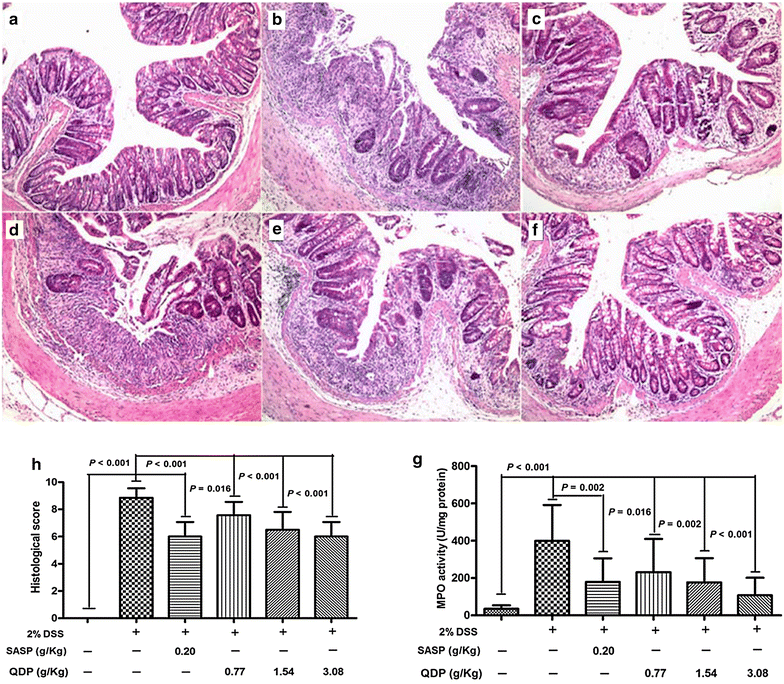
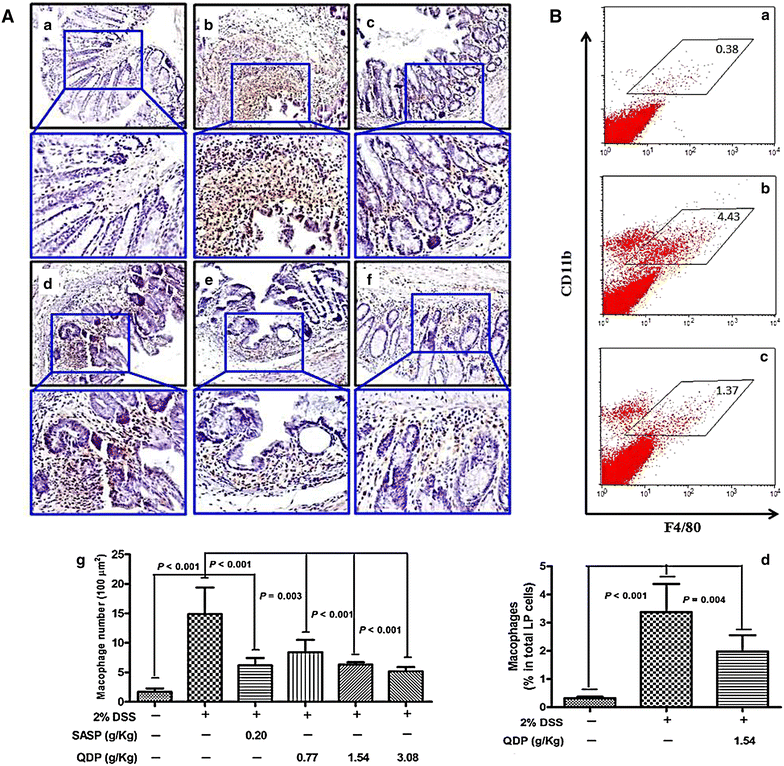
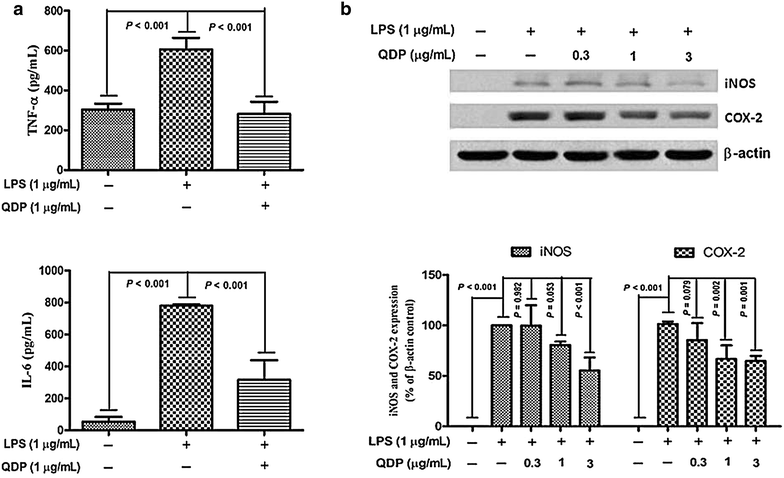
Methods: Seven- to eight-week-old male C57BL/6 mice were challenged with 2.0 % DSS in drinking water for 5 days and then the colitic mice were arbitrarily allocated into five groups (n = 10 for each group). QDP (0.77, 1.54 and 3.08 g/kg) and sulfasalazine (SASP) (0.20 g/kg) were orally administered for 7 days. The disease activity index was determined by scores of body weight loss, diarrhea and rectal bleeding; histological signs of damage was analyzed by H&E staining; myeloperoxidase activity was measured by colorimetric method, levels of proinflammatory cytokines were determined by ELISA; changes in macrophages in the colon were analyzed by immunohistochemistry (IHC) and flow cytometry. Lipopolysaccharide (LPS)-induced RAW264.7 cells were treated with or without QDP, then the production of TNF-α and IL-6 were measured by ELISA; and protein molecules such as COX-2, iNOS, IкB-α were determined by Western blot.
Results: Oral administration of QDP at dosages of 1.54 and 3.08 g/kg significantly reduced disease activity index on day 12 (P < 0.001 for 1.54 g/kg and P < 0.0008 for 3.08 g/kg), colon shortening (P = 0.012 for 1.54 g/kg, P = 0.001 for 3.08 g/kg), histological damage (P < 0.001 for 1.54 g/kg, P < 0.001 for 3.08 g/kg) and colonic myeloperoxidase activity (P = 0.002 for 1.54 g/kg, P < 0.001 for 3.08 g/kg) of DSS-treated mice. Moreover, QDP treatment (1.54 and 3.08 g/kg) significantly decreased DSS-induced infiltration of macrophages, and production of TNF-α (P = 0.005 for 1.54 g/kg, P = 0.002 for 3.08 g/kg), IL-1β (P = 0.008 for 1.54 g/kg, P = 0.002 for 3.08 g/kg) and IL-6 (P = 0.011 for 1.54 g/kg, P = 0.004 for 3.08 g/kg) in colonic tissues, and also reduced serum MCP-1 levels (P = 0.001 for 1.54 g/kg, P < 0.001 for 3.08 g/kg). In RAW264.7 cells, QDP significantly suppressed LPS-induced production of TNF-α and IL-6 (Both P < 0.001 for 1.0 μg/mL QDP treatment) and expression levels of COX-2 (P = 0.002 and P = 0.001 for 1 and 3 μg/mL QDP treatment, respectively) and iNOS (P < 0.001 for 3 μg/mL QDP treatment) by inhibiting IкB-α degradation (P = 0.007 and P = 0.004 for 1 and 3 μg/mL QDP treatment, respectively) and NF-кB p65 nuclear translocation.
Conclusion: QDP suppressed the inflammatory responses of colonic macrophages in DSS-induced UC in mice and LPS-induced RAW264.7 cells.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

