Astrogliosis Induced by Brain Injury Is Regulated by Sema4B Phosphorylation
- PMID: 26464987
- PMCID: PMC4586933
- DOI: 10.1523/ENEURO.0078-14.2015
Astrogliosis Induced by Brain Injury Is Regulated by Sema4B Phosphorylation
Abstract
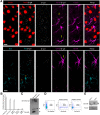
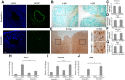
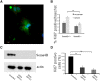
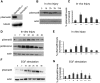
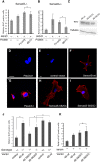
Injury to the CNS induces astrogliosis, an astrocyte-mediated response that has both beneficial and detrimental impacts on surrounding neural and non-neural cells. The precise signaling events underlying astrogliosis are not fully characterized. Here, we show that astrocyte activation was altered and proliferation was reduced in Semaphorin 4B (Sema4B)-deficient mice following injury. Proliferation of cultured Sema4B(-/-) astrocytes was also significantly reduced. In contrast to its expected role as a ligand, the Sema4B ectodomain was not able to rescue Sema4B(-/-) astrocyte proliferation but instead acted as an antagonist against Sema4B(+/-) astrocytes. Furthermore, the effects of Sema4B on astrocyte proliferation were dependent on phosphorylation of the intracellular domain at Ser825. Our results suggest that Sema4B functions as an astrocyte receptor, defining a novel signaling pathway that regulates astrogliosis after CNS injury.
Keywords: CNS injury; Sema4B; astrogliosis.
Figures


References
-
- Bardehle S, Krüger M, Buggenthin F, Schwausch J, Ninkovic J, Clevers H, Snippert HJ, Theis FJ, Meyer-Luehmann M, Bechmann I, Dimou L, Götz M (2013) Live imaging of astrocyte responses to acute injury reveals selective juxtavascular proliferation. Nat Neurosci 16:580–586. 10.1038/nn.3371 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
