Water desalination with a single-layer MoS2 nanopore
- PMID: 26465062
- PMCID: PMC4634321
- DOI: 10.1038/ncomms9616
Water desalination with a single-layer MoS2 nanopore
Abstract
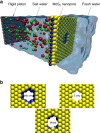
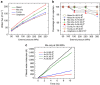
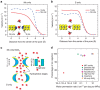
Efficient desalination of water continues to be a problem facing the society. Advances in nanotechnology have led to the development of a variety of nanoporous membranes for water purification. Here we show, by performing molecular dynamics simulations, that a nanopore in a single-layer molybdenum disulfide can effectively reject ions and allow transport of water at a high rate. More than 88% of ions are rejected by membranes having pore areas ranging from 20 to 60 Å(2). Water flux is found to be two to five orders of magnitude greater than that of other known nanoporous membranes. Pore chemistry is shown to play a significant role in modulating the water flux. Pores with only molybdenum atoms on their edges lead to higher fluxes, which are ∼ 70% greater than that of graphene nanopores. These observations are explained by permeation coefficients, energy barriers, water density and velocity distributions in the pores.
Figures

References
-
- Elimelech M. & Phillip W. A. The future of seawater desalination: energy, technology, and the environment. Science 333, 712–717 (2011). - PubMed
-
- Zhao S. F., Zou L., Tang C. Y. Y. & Mulcahy D. Recent developments in forward osmosis: opportunities and challenges. J. Membr. Sci. 396, 1–21 (2012).
-
- Shannon M. A. et al.. Science and technology for water purification in the coming decades. Nature 452, 301–310 (2008). - PubMed
-
- Fritzmann C., Lowenberg J., Wintgens T. & Melin T. State-of-the-art of reverse osmosis desalination. Desalination 216, 1–76 (2007).
-
- Khawaji A. D., Kutubkhanah I. K. & Wie J. M. Advances in seawater desalination technologies. Desalination 221, 47–69 (2008).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

