A Randomized 2x2 Factorial Clinical Trial of Renal Transplantation: Steroid-Free Maintenance Immunosuppression with Calcineurin Inhibitor Withdrawal after Six Months Associates with Improved Renal Function and Reduced Chronic Histopathology
- PMID: 26465152
- PMCID: PMC4605789
- DOI: 10.1371/journal.pone.0139247
A Randomized 2x2 Factorial Clinical Trial of Renal Transplantation: Steroid-Free Maintenance Immunosuppression with Calcineurin Inhibitor Withdrawal after Six Months Associates with Improved Renal Function and Reduced Chronic Histopathology
Abstract
Introduction: The two most significant impediments to renal allograft survival are rejection and the direct nephrotoxicity of the immunosuppressant drugs required to prevent it. Calcineurin inhibitors (CNI), a mainstay of most immunosuppression regimens, are particularly nephrotoxic. Until less toxic antirejection agents become available, the only option is to optimize our use of those at hand.
Aim: To determine whether intensive rabbit anti-thymocyte globulin (rATG) induction followed by CNI withdrawal would individually or combined improve graft function and reduce graft chronic histopathology-surrogates for graft and, therefore, patient survival. As previously reported, a single large rATG dose over 24 hours was well-tolerated and associated with better renal function, fewer infections, and improved patient survival. Here we report testing whether complete CNI discontinuation would improve renal function and decrease graft pathology.
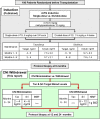
Methods: Between April 20, 2004 and 4-14-2009 we conducted a prospective, randomized, non-blinded renal transplantation trial of two rATG dosing protocols (single dose, 6 mg/kg vs. divided doses, 1.5 mg/kg every other day x 4; target enrollment = 180). Subsequent maintenance immunosuppression consisted of tacrolimus, a CNI, and sirolimus, a mammalian target of rapamycin inhibitor. We report here the outcome of converting patients after six months either to minimized tacrolimus/sirolimus or mycophenolate mofetil/sirolimus. Primary endpoints were graft function and chronic histopathology from protocol kidney biopsies at 12 and 24 months.
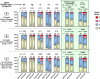
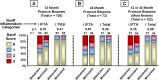
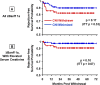
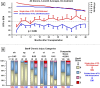
Results: CNI withdrawal (on-treatment analysis) associated with better graft function (p <0.001) and lower chronic histopathology composite scores in protocol biopsies at 12 (p = 0.003) and 24 (p = 0.013) months, without affecting patient (p = 0.81) or graft (p = 0.93) survival, or rejection rate (p = 0.17).
Conclusion: CNI (tacrolimus) withdrawal at six months may provide a strategy for decreased nephrotoxicity and improved long-term function in steroid-free low immunological risk renal transplant patients.
Trial registration: ClinicalTrials.gov NCT00556933.
Conflict of interest statement
Figures



References
-
- Woodle ES, First MR, Pirsch J, Shihab F, Gaber AO, Van Veldhuisen P. A prospective, randomized, double-blind, placebo-controlled multicenter trial comparing early (7 day) corticosteroid cessation versus long-term, low-dose corticosteroid therapy. Ann Surg. 2008;248(4):564–77. 10.1097/SLA.0b013e318187d1da - DOI - PubMed
-
- Kasiske BL, Gaston RS, Gourishankar S, Halloran PF, Matas AJ, Jeffery J, et al. Long-term deterioration of kidney allograft function. Am J Transplant. 2005;5(6):1405–14. . - PubMed
-
- Meier-Kriesche HU, Schold JD, Srinivas TR, Kaplan B. Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant. 2004;4(3):378–83. . - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

