Presence of Tablet Remnants of Nevirapine Extended-Release in Stools and Its Impact on Virological Outcome in HIV-1-Infected Patients: A Prospective Cohort Study
- PMID: 26465325
- PMCID: PMC4605833
- DOI: 10.1371/journal.pone.0140574
Presence of Tablet Remnants of Nevirapine Extended-Release in Stools and Its Impact on Virological Outcome in HIV-1-Infected Patients: A Prospective Cohort Study
Abstract
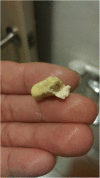
Background: Nevirapine extended-release (NVP-XR) taken once daily remains an effective antiretroviral agent for patients infected with HIV-1 strains that do not harbor resistance mutations. Presence of tablet remnants of NVP XR in stools was reported in 1.19% and 3.05% of subjects in two clinical trials. However, the prevalence may have been underestimated because the information was retrospectively collected in the studies.
Methods: Between April and December 2014, we prospectively inquired about the frequency of noticing tablet remnants of NVP XR in stools in HIV-1-infected patients who switched to antiretroviral regimens containing NVP XR plus 2 nucleos(t)ide reverse-transcriptase inhibitors. Patients were invited to participate in therapeutic drug monitoring of plasma concentrations of NVP 12 or 24 hours after taking the previous dose (C12 and C24, respectively) of NVP XR using high-performance liquid chromatography. The information on clinical characteristics, including plasma HIV RNA load and CD4 lymphocyte count, at baseline and during follow-up was recorded.
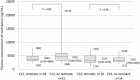
Results: During the 9-month study period, 272 patients switched to NVP XR-based regimens and 60 (22.1%) noticed tablet remnants of NVP XR in stools, in whom 54.2% reported noticing the tablet remnants at least once weekly. Compared with patients who did not notice tablet remnants, those who noticed tablet remnants had a higher mean CD4 lymphocyte count (629 vs 495 cells/mm3, P = 0.0002) and a similar mean plasma HIV RNA load (1.57 vs 1.61 log10 copies/mL, P = 0.76) on switch. At about 12 and 24 weeks after switch, patients who noticed tablet remnants continued to have a similar mean plasma HIV RNA load (1.39 vs 1.43 log10 copies/mL, P = 0.43; and 1.30 vs 1.37 log10 copies/mL, P = 0.26, respectively), but had a lower median NVP C12 (3640 vs 4730 ng/mL, P = 0.06), and a similar median NVP C24 (3220 vs 3330 ng/ml, P = 0.95) when compared with those who did not notice tablet remnants.
Conclusions: The presence of tablet remnants of NVP XR in stools is not uncommon in HIV-1-infected Taiwanese patients receiving NVP XR-based antiretroviral regimens, which does not have an adverse impact on the virological and immunological outcomes.
Conflict of interest statement
Figures
References
-
- Guideline for the Use of Antiretroviral Agents in HIV-1-infected Adults and Aldolescents. Department of Health and Human Services; Available at: https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf Accessed: June 6, 2015
-
- van Leth F, Phanuphak P, Ruxrungtham K, Baraldi E, Miller S, Gazzard B, et al. Comparison of first-line antiretroviral therapy with regimens including nevirapine, efavirenz, or both drugs, plus stavudine and lamivudine: a randomised open-label trial, the 2NN Study. Lancet 2004; 363: 1253–63. - PubMed
-
- Kappelhoff BS, Huitema AD, van Leth F, Robinson PA, MacGregor TR, Lange JM, et al. 2NN study group. Pharmacokinetics of nevirapine: once daily versus twice daily dosing in the 2NN study. HIV Clin Trials 2005: 6; 254–61. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

