Emergency Department Visits for Adverse Events Related to Dietary Supplements
- PMID: 26465986
- PMCID: PMC6196363
- DOI: 10.1056/NEJMsa1504267
Emergency Department Visits for Adverse Events Related to Dietary Supplements
Abstract
Background: Dietary supplements, such as herbal or complementary nutritional products and micronutrients (vitamins and minerals), are commonly used in the United States, yet national data on adverse effects are limited.
Methods: We used nationally representative surveillance data from 63 emergency departments obtained from 2004 through 2013 to describe visits to U.S. emergency departments because of adverse events related to dietary supplements.
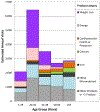
Results: On the basis of 3667 cases, we estimated that 23,005 (95% confidence interval [CI], 18,611 to 27,398) emergency department visits per year were attributed to adverse events related to dietary supplements. These visits resulted in an estimated 2154 hospitalizations (95% CI, 1342 to 2967) annually. Such visits frequently involved young adults between the ages of 20 and 34 years (28.0% of visits; 95% CI, 25.1 to 30.8) and unsupervised children (21.2% of visits; 95% CI, 18.4 to 24.0). After the exclusion of unsupervised ingestion of dietary supplements by children, 65.9% (95% CI, 63.2 to 68.5) of emergency department visits for single-supplement-related adverse events involved herbal or complementary nutritional products; 31.8% (95% CI, 29.2 to 34.3) involved micronutrients. Herbal or complementary nutritional products for weight loss (25.5%; 95% CI, 23.1 to 27.9) and increased energy (10.0%; 95% CI, 8.0 to 11.9) were commonly implicated. Weight-loss or energy products caused 71.8% (95% CI, 67.6 to 76.1) of supplement-related adverse events involving palpitations, chest pain, or tachycardia, and 58.0% (95% CI, 52.2 to 63.7) involved persons 20 to 34 years of age. Among adults 65 years of age or older, choking or pill-induced dysphagia or globus caused 37.6% (95% CI, 29.1 to 46.2) of all emergency department visits for supplement-related adverse events; micronutrients were implicated in 83.1% (95% CI, 73.3 to 92.9) of these visits.
Conclusions: An estimated 23,000 emergency department visits in the United States every year are attributed to adverse events related to dietary supplements. Such visits commonly involve cardiovascular manifestations from weight-loss or energy products among young adults and swallowing problems, often associated with micronutrients, among older adults. (Funded by the Department of Health and Human Services.).
Figures

Comment in
-
Emergency department visits and hospitalisations for adverse events related to dietary supplements are common.Evid Based Med. 2016 Apr;21(2):79. doi: 10.1136/ebmed-2015-110362. Epub 2016 Jan 22. Evid Based Med. 2016. PMID: 26801051 No abstract available.
-
Emergency Department Visits Related to Dietary Supplements.N Engl J Med. 2016 Feb 18;374(7):695. doi: 10.1056/NEJMc1514454. N Engl J Med. 2016. PMID: 26886539 No abstract available.
-
Emergency Department Visits Related to Dietary Supplements.N Engl J Med. 2016 Feb 18;374(7):694-5. doi: 10.1056/NEJMc1514454. N Engl J Med. 2016. PMID: 26886540 No abstract available.
-
[Dietary supplements - blessing or curse?].Praxis (Bern 1994). 2016 Mar 2;105(5):289-90. doi: 10.1024/1661-8157/a002267. Praxis (Bern 1994). 2016. PMID: 26934015 German. No abstract available.
References
-
- Dietary Supplement Health and Education Act of 1994. Pub L No. 103–417, 108 Stat 4325.
-
- Bailey RL, Gahche JJ, Miller PE, Thomas PR, Dwyer JT. Why US adults use dietary supplements. JAMA Intern Med 2013;173:355–61. - PubMed
-
- US Department of Health and Human Services, Office of Disease Prevention and Health Promotion. Report of the Commission on Dietary Supplement Labels. (Accessed November 25, 2014, at http://web.health.gov/dietsupp/final.pdf.)
-
- US Government Accountability Office. Dietary Supplements: FDA may have opportunities to expand its use of reported health problems to oversee products (GAO-13–244). March 18, 2013. (Accessed January 10, 2015, at http://www.gao.gov/assets/660/653113.pdf.)
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
