Increased Oxidative and Nitrative Stress Accelerates Aging of the Retinal Vasculature in the Diabetic Retina
- PMID: 26466127
- PMCID: PMC4605485
- DOI: 10.1371/journal.pone.0139664
Increased Oxidative and Nitrative Stress Accelerates Aging of the Retinal Vasculature in the Diabetic Retina
Abstract
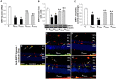
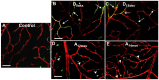
Hyperglycemia-induced retinal oxidative and nitrative stress can accelerate vascular cell aging, which may lead to vascular dysfunction as seen in diabetes. There is no information on whether this may contribute to the progression of diabetic retinopathy (DR). In this study, we have assessed the occurrence of senescence-associated markers in retinas of streptozotocin-induced diabetic rats at 8 and 12 weeks of hyperglycemia as compared to normoglycemic aging (12 and 14 months) and adult (4.5 months) rat retinas. We have found that in the diabetic retinas there was an up-regulation of senescence-associated markers SA-β-Gal, p16INK4a and miR34a, which correlated with decreased expression of SIRT1, a target of miR34a. Expression of senescence-associated factors primarily found in retinal microvasculature of diabetic rats exceeded levels measured in adult and aging rat retinas. In aging rats, retinal expression of senescence associated-factors was mainly localized at the level of the retinal pigmented epithelium and only minimally in the retinal microvasculature. The expression of oxidative/nitrative stress markers such as 4-hydroxynonenal and nitrotyrosine was more pronounced in the retinal vasculature of diabetic rats as compared to normoglycemic aging and adult rat retinas. Treatments of STZ-rats with the anti-nitrating drug FeTPPS (10mg/Kg/day) significantly reduced the appearance of senescence markers in the retinal microvasculature. Our results demonstrate that hyperglycemia accelerates retinal microvascular cell aging whereas physiological aging affects primarily cells of the retinal pigmented epithelium. In conclusion, hyperglycemia-induced retinal vessel dysfunction and DR progression involve vascular cell senescence due to increased oxidative/nitrative stress.
Conflict of interest statement
Figures








References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

