Oxidative stress inhibits distant metastasis by human melanoma cells
- PMID: 26466563
- PMCID: PMC4644103
- DOI: 10.1038/nature15726
Oxidative stress inhibits distant metastasis by human melanoma cells
Abstract
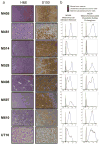
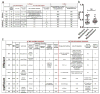
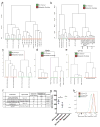
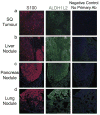
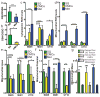
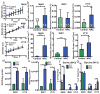
Solid cancer cells commonly enter the blood and disseminate systemically, but are highly inefficient at forming distant metastases for poorly understood reasons. Here we studied human melanomas that differed in their metastasis histories in patients and in their capacity to metastasize in NOD-SCID-Il2rg(-/-) (NSG) mice. We show that melanomas had high frequencies of cells that formed subcutaneous tumours, but much lower percentages of cells that formed tumours after intravenous or intrasplenic transplantation, particularly among inefficiently metastasizing melanomas. Melanoma cells in the blood and visceral organs experienced oxidative stress not observed in established subcutaneous tumours. Successfully metastasizing melanomas underwent reversible metabolic changes during metastasis that increased their capacity to withstand oxidative stress, including increased dependence on NADPH-generating enzymes in the folate pathway. Antioxidants promoted distant metastasis in NSG mice. Folate pathway inhibition using low-dose methotrexate, ALDH1L2 knockdown, or MTHFD1 knockdown inhibited distant metastasis without significantly affecting the growth of subcutaneous tumours in the same mice. Oxidative stress thus limits distant metastasis by melanoma cells in vivo.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



Comment in
-
Cancer: The enemy of my enemy is my friend.Nature. 2015 Nov 12;527(7577):170-1. doi: 10.1038/nature15644. Epub 2015 Oct 21. Nature. 2015. PMID: 26503052 No abstract available.
-
Metabolism in melanoma metastasis.Pigment Cell Melanoma Res. 2016 Mar;29(2):118-9. doi: 10.1111/pcmr.12440. Epub 2015 Dec 18. Pigment Cell Melanoma Res. 2016. PMID: 26575789 No abstract available.
-
Metastasis and Oxidative Stress: Are Antioxidants a Metabolic Driver of Progression?Cell Metab. 2015 Dec 1;22(6):956-8. doi: 10.1016/j.cmet.2015.11.008. Cell Metab. 2015. PMID: 26636492
References
-
- Yu M, Bardia A, Wittner BS, Stott SL, Smas ME, Ting DT, Isakoff SJ, Ciciliano JC, Wells MN, Shah AM, Concannon KF, Donaldson MC, Sequist LV, Brachtel E, Sgroi D, Baselga J, Ramaswamy S, Toner M, Haber DA, Maheswaran S. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 2013;339:580–584. - PMC - PubMed
-
- Stott SL, Lee RJ, Nagrath S, Yu M, Miyamoto DT, Ulkus L, Inserra EJ, Ulman M, Springer S, Nakamura Z, Moore AL, Tsukrov DI, Kempner ME, Dahl DM, Wu CL, Iafrate AJ, Smith MR, Tompkins RG, Sequist LV, Toner M, Haber DA, Maheswaran S. Isolation and characterization of circulating tumor cells from patients with localized and metastatic prostate cancer. Science Translational Medicine. 2010;2:25ra23. - PMC - PubMed
-
- Yu M, Bardia A, Aceto N, Bersani F, Madden MW, Donaldson MC, Desai R, Zhu H, Comaills V, Zheng Z, Wittner BS, Stojanov P, Brachtel E, Sgroi D, Kapur R, Shioda T, Ting DT, Ramaswamy S, Getz G, Iafrate AJ, Benes C, Toner M, Maheswaran S, Haber DA. Cancer therapy. Ex vivo culture of circulating breast tumor cells for individualized testing of drug susceptibility. Science. 2014;345:216–220. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

