Red Blood Cell Dysfunction Induced by High-Fat Diet: Potential Implications for Obesity-Related Atherosclerosis
- PMID: 26467254
- PMCID: PMC4772773
- DOI: 10.1161/CIRCULATIONAHA.115.017313
Red Blood Cell Dysfunction Induced by High-Fat Diet: Potential Implications for Obesity-Related Atherosclerosis
Abstract
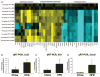
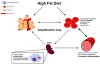
Background: High-fat diet (HFD) promotes endothelial dysfunction and proinflammatory monocyte activation, which contribute to atherosclerosis in obesity. We investigated whether HFD also induces the dysfunction of red blood cells (RBCs), which serve as a reservoir for chemokines via binding to Duffy antigen receptor for chemokines (DARC).
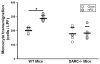
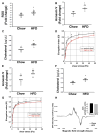
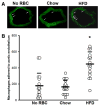
Methods and results: A 60% HFD for 12 weeks, which produced only minor changes in lipid profile in C57/BL6 mice, markedly augmented the levels of monocyte chemoattractant protein-1 bound to RBCs, which in turn stimulated macrophage migration through an endothelial monolayer. Levels of RBC-bound KC were also increased by HFD. These effects of HFD were abolished in DARC(-/-) mice. In RBCs from HFD-fed wild-type and DARC(-/-) mice, levels of membrane cholesterol and phosphatidylserine externalization were increased, fostering RBC-macrophage inflammatory interactions and promoting macrophage phagocytosis in vitro. When labeled ex vivo and injected into wild-type mice, RBCs from HFD-fed mice exhibited ≈3-fold increase in splenic uptake. Finally, RBCs from HFD-fed mice induced increased macrophage adhesion to the endothelium when they were incubated with isolated aortic segments, indicating endothelial activation.
Conclusions: RBC dysfunction, analogous to endothelial dysfunction, occurs early during diet-induced obesity and may serve as a mediator of atherosclerosis. These findings may have implications for the pathogenesis of atherosclerosis in obesity, a worldwide epidemic.
Keywords: atherosclerosis; erythrocytes; leukocytes; obesity.
© 2015 American Heart Association, Inc.
Figures


Comment in
-
Atherothrombosis: Seeing Red?Circulation. 2015 Nov 17;132(20):1860-2. doi: 10.1161/CIRCULATIONAHA.115.019259. Epub 2015 Oct 14. Circulation. 2015. PMID: 26467255 Free PMC article. No abstract available.
References
-
- Gower RM, Wu H, Foster GA, Devaraj S, Jialal I, Ballantyne CM, Knowlton AA, Simon SI. CD11c/CD18 expression is upregulated on blood monocytes during hypertriglyceridemia and enhances adhesion to vascular cell adhesion molecule-1. Arterioscler Thromb Vasc Biol. 2011;31:160–166. doi: 10.1161/ATVBAHA.110.215434. - DOI - PMC - PubMed
-
- Rothe G, Herr AS, Stöhr J, Abletshauser C, Weidinger G, Schmitz G. A more mature phenotype of blood mononuclear phagocytes is induced by fluvastatin treatment in hypercholesterolemic patients with coronary heart disease. Atherosclerosis. 1999;144:251–261. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

