Complementary isonitrile-based multicomponent reactions for the synthesis of diversified cytotoxic hemiasterlin analogues
- PMID: 26467486
- PMCID: PMC4685954
- DOI: 10.1039/c5ob01882j
Complementary isonitrile-based multicomponent reactions for the synthesis of diversified cytotoxic hemiasterlin analogues
Abstract
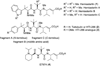
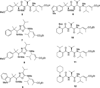
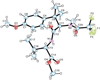
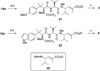
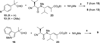
A small family of structural analogues of the antimitotic tripeptides, hemiasterlins, have been designed and synthesized as potential inhibitors of tubulin polymerization. The effectiveness of a multicomponent approach was fully demonstrated by applying complementary versions of the isocyanide-based Ugi reaction. Compounds strictly related to the lead natural products, as well as more extensively modified analogues, have been synthesized in a concise and convergent manner. In some cases, biological evaluation provided evidence for strong cytotoxic activity (six human tumor cell lines) and for potent inhibition of tubulin polymerization.
Figures

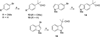

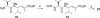
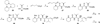
References
-
- Pando O, Stark S, Denkert A, Porzel A, Preusentanz R, Wessjohann LA. J. Am. Chem. Soc. 2011;133:7692. - PubMed
-
- Stucchi M, Cairati S, Cetin-Atalay R, Christodoulou MS, Grazioso G, Pescitelli G, Silvani A, Yildirim DC, Lesma G. Org. Biomol. Chem. 2015;13:4993. - PubMed
- Lesma G, Meneghetti F, Sacchetti A, Stucchi M, Silvani A. Beilstein J. Org. Chem. 2014;10:1383. - PMC - PubMed
- Silvani A, Lesma G, Crippa S, Vece V. Tetrahedron. 2014;70:3994.
- Lesma G, Cecchi R, Crippa S, Giovanelli P, Meneghetti F, Musolino M, Sacchetti A, Silvani A. Org. Biomol. Chem. 2012;10:9004. - PubMed
-
- Gamble WR, Durso NA, Fuller RW, Westergaard CK, Johnson TR, Sackett DL, Hamel E, Cardellina II JH, Boyd MR. Bioorg. Med. Chem. 1999;7:1611. - PubMed
- Talpir R, Benayahu Y, Kashman Y, Pannell L, Schleyer M. Tetrahedron Lett. 1994;35:4453.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

