Multisite HPV16/18 Vaccine Efficacy Against Cervical, Anal, and Oral HPV Infection
- PMID: 26467666
- PMCID: PMC4862406
- DOI: 10.1093/jnci/djv302
Multisite HPV16/18 Vaccine Efficacy Against Cervical, Anal, and Oral HPV Infection
Abstract
Background: Previous Costa Rica Vaccine Trial (CVT) reports separately demonstrated vaccine efficacy against HPV16 and HPV18 (HPV16/18) infections at the cervical, anal, and oral regions; however, the combined overall multisite efficacy (protection at all three sites) and vaccine efficacy among women infected with HPV16 or HPV18 prior to vaccination are less known.
Methods: Women age 18 to 25 years from the CVT were randomly assigned to the HPV16/18 vaccine (Cervarix) or a hepatitis A vaccine. Cervical, oral, and anal specimens were collected at the four-year follow-up visit from 4186 women. Multisite and single-site vaccine efficacies (VEs) and 95% confidence intervals (CIs) were computed for one-time detection of point prevalent HPV16/18 in the cervical, anal, and oral regions four years after vaccination. All statistical tests were two-sided.
Results: The multisite woman-level vaccine efficacy was highest among "naïve" women (HPV16/18 seronegative and cervical HPV high-risk DNA negative at vaccination) (vaccine efficacy = 83.5%, 95% CI = 72.1% to 90.8%). Multisite woman-level vaccine efficacy was also demonstrated among women with evidence of a pre-enrollment HPV16 or HPV18 infection (seropositive for HPV16 and/or HPV18 but cervical HPV16/18 DNA negative at vaccination) (vaccine efficacy = 57.8%, 95% CI = 34.4% to 73.4%), but not in those with cervical HPV16 and/or HPV18 DNA at vaccination (anal/oral HPV16/18 VE = 25.3%, 95% CI = -40.4% to 61.1%). Concordant HPV16/18 infections at two or three sites were also less common in HPV16/18-infected women in the HPV vaccine vs control arm (7.4% vs 30.4%, P < .001).
Conclusions: This study found high multisite vaccine efficacy among "naïve" women and also suggests the vaccine may provide protection against HPV16/18 infections at one or more anatomic sites among some women infected with these types prior to HPV16/18 vaccination.
Trial registration: ClinicalTrials.gov NCT00128661.
Published by Oxford University Press 2015. This work is written by (a) US Government employee(s) and is in the public domain in the US.
Figures
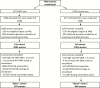
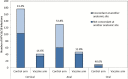
Comment in
-
HPV Vaccine Confers Multisite Protection.Cancer Discov. 2015 Jun;5(6):OF1. doi: 10.1158/2159-8290.CD-NB2015-060. Epub 2015 Apr 22. Cancer Discov. 2015. PMID: 25904655
References
-
- Kjaer SK, Sigurdsson K, Iversen OE, et al. A pooled analysis of continued prophylactic efficacy of quadrivalent human papillomavirus (Types 6/11/16/18) vaccine against high-grade cervical and external genital lesions. Cancer Prev Res (Phila). 2009;2 (10):868–878. - PubMed
-
- Lehtinen M, Paavonen J, Wheeler CM, et al. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2012;13 (1):89–99. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

