Direct improvement of quality of life in colorectal cancer patients using a tailored pathway with quality of life diagnosis and therapy (DIQOL): study protocol for a randomised controlled trial
- PMID: 26467994
- PMCID: PMC4607244
- DOI: 10.1186/s13063-015-0972-y
Direct improvement of quality of life in colorectal cancer patients using a tailored pathway with quality of life diagnosis and therapy (DIQOL): study protocol for a randomised controlled trial
Abstract
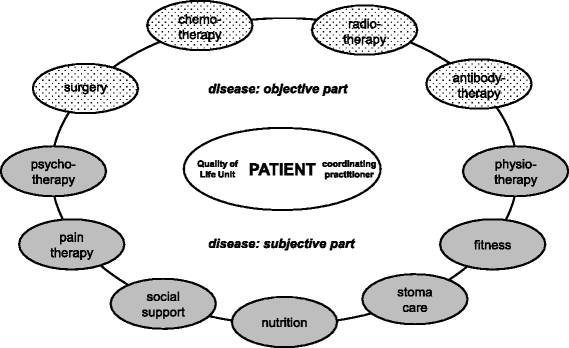
Background: Medical treatment in patient-centred care in oncology is broadly managed and regulated in terms of guideline development, cancer centres, and quality assurance by cancer registries. In contrast to this quality management cycle (PDCA), there are no equal standards for patient-reported outcomes like quality of life (QoL). Therefore, the Tumour Centre Regensburg e.V., a population-based regional cancer registry covering a population of more than 2.2 million people in the Upper Palatinate and Lower Bavaria, Germany, designed and implemented a QoL pathway. In a complex intervention with QoL diagnosis and therapy (multidimensional therapeutic network), effectiveness for patients with breast cancer has been demonstrated. To provide local tailored QoL diagnosis and therapy to other cancer patients as well, external validity needs to be extended by adapting the QoL pathway to another tumour entity.
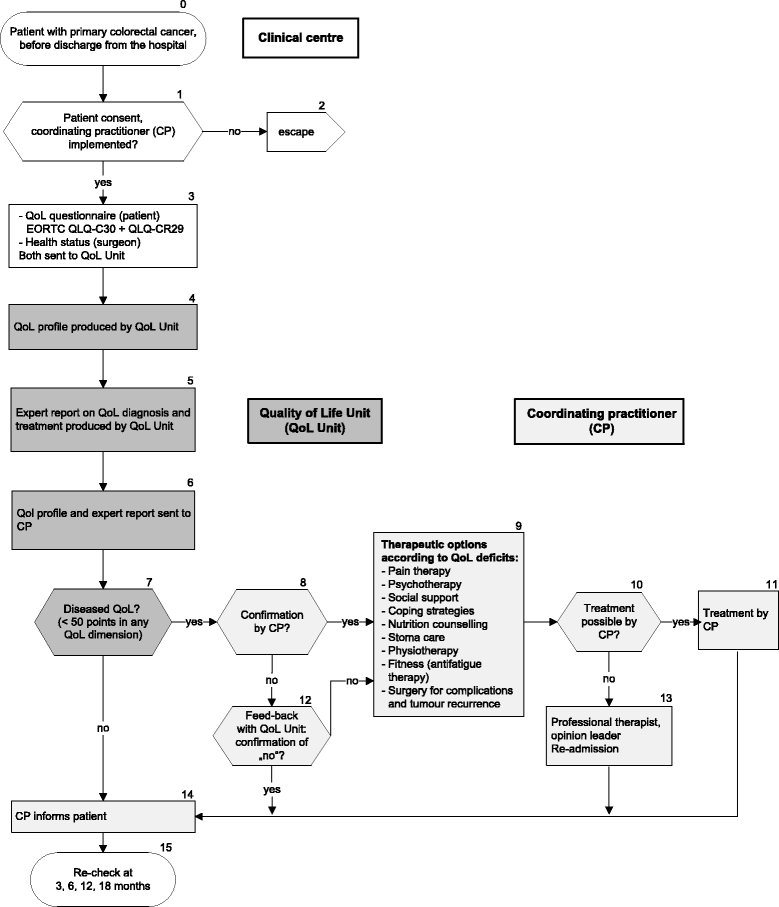
Methods/design: The QoL pathway will be tested for colorectal cancer patients in a pragmatic randomised controlled trial. Two hundred twenty primary colorectal cancer patients, surgically treated in one of four hospitals, will be included. QoL is measured in all patients 0, 3, 6, 12, and 18 months after surgery (European Organisation for Research and Treatment of Cancer (EORTC) QLQ-C30, QLQ-CR29). In the intervention group, QoL scores are transformed into a QoL profile. This is sent to the coordinating practitioner (general practitioner, internist, or oncologist) with an expert report including treatment recommendations for QoL deficits. The control group receives routine follow-up care attending the guideline recommendations for colorectal cancer without profile or expert report. At the primary endpoint (12 months), the rates of patients with diseased QoL in both groups are compared.
Discussion: This randomised trial is the first complex intervention investigating the effectiveness of an intervention with QoL diagnosis and tailored QoL therapy in colorectal cancer patients. The results will show if this QoL pathway improves the patients' QoL during follow-up care of their disease.
Trial registration: ClinicalTrials.gov, NCT02321813 (registered December 2014).
Figures



References
-
- Hollingworth W, Metcalfe C, Mancero S, Harris S, Campbell C, Biddle L, et al. Are needs assessments cost effective in reducing distress among patients with cancer? A randomized controlled trial using the distress thermometer and problem list. J Clin Oncol. 2013;31:3631–8. doi: 10.1200/JCO.2012.48.3040. - DOI - PubMed
-
- Klinkhammer-Schalke M, Koller M, Wyatt JC, Steinger B, Ehret C, Ernst B, et al. Quality of life diagnosis and therapy as complex intervention for improvement of health in breast cancer patients: delineating the conceptual, methodological, and logistic requirements (modeling) Langenbecks Arch Surg. 2008;393:1–12. doi: 10.1007/s00423-007-0210-5. - DOI - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

