Capecitabine in combination with either cisplatin or weekly paclitaxel as a first-line treatment for metastatic esophageal squamous cell carcinoma: a randomized phase II study
- PMID: 26468007
- PMCID: PMC4606554
- DOI: 10.1186/s12885-015-1716-9
Capecitabine in combination with either cisplatin or weekly paclitaxel as a first-line treatment for metastatic esophageal squamous cell carcinoma: a randomized phase II study
Abstract
Background: The aim of this study was to assess the efficacy and safety of a combination regimen of capecitabine plus cisplatin (CC) or capecitabine plus paclitaxel (CP) as a first-line treatment in patients with metastatic esophageal squamous cell carcinoma.
Methods: Patients with recurrent or metastatic esophageal squamous cell carcinoma were enrolled in this open-label, phase II, randomized trial. Patients were assigned to either the CC arm (days [D]1-14 capecitabine 1000 mg/m(2) twice daily + D1 cisplatin 75 mg/m(2), every 3 weeks) or the CP arm (D1-14 capecitabine 1000 mg/m(2) twice daily + D1, 8 paclitaxel 80 mg/m(2), every 3 weeks). The primary endpoint of the study was response rate and secondary endpoints were progression-free survival (PFS), overall survival (OS), toxicity and quality of life.
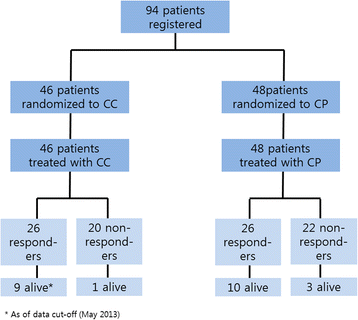
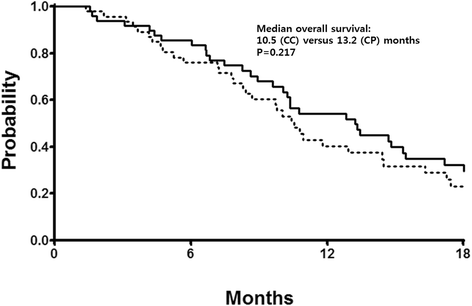
Results: A total of 94 patients were entered into this study between October 2008 and October 2012, 46 patients in the CC arm and 48 in the CP arm. Patients in both arms received a median of six cycles of treatment (range, 1-14) and the response rates were 57 and 58 % in the cisplatin and paclitaxel arm, respectively. With a median follow-up of 23 months, the median PFS was 5.1 months (95 % CI 4.0-6.2 months) in the cisplatin arm and 6.7 months (95 % CI 4.9-8.5 months) in the paclitaxel arm, whereas the median OS was 10.5 months (95 % CI 9.2-11.9 months) in the cisplatin arm and 13.2 months (95 % CI 9.4-17.0 months) in the paclitaxel arm. Patients in the cisplatin arm were more likely to experience neutropenia and thrombocytopenia, whereas patients in the paclitaxel arm had a higher frequency of neuropathy and alopecia. Quality of life was similar between treatment arms.
Conclusions: Both CC and CP regimens were effective and well tolerated as a first-line treatment in patients with metastatic esophageal squamous cell carcinoma.
Figures

References
-
- Enzinger PC, Ilson DH, Kelsen DP. Chemotherapy in esophageal cancer. Semin Oncol. 1999;26:12–20. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

