Macrophages mediate flagellin induced inflammasome activation and host defense in zebrafish
- PMID: 26468080
- PMCID: PMC5027955
- DOI: 10.1111/cmi.12536
Macrophages mediate flagellin induced inflammasome activation and host defense in zebrafish
Abstract
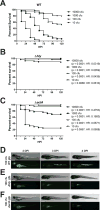
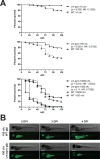
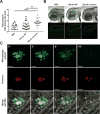
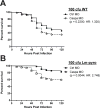
The inflammasome is an innate immune complex whose rapid inflammatory outputs play a critical role in controlling infection; however, the host cells that mediate inflammasome responses in vivo are not well defined. Using zebrafish larvae, we examined the cellular immune responses to inflammasome activation during infection. We compared the host responses with two Listeria monocytogenes strains: wild type and Lm-pyro, a strain engineered to activate the inflammasome via ectopic expression of flagellin. Infection with Lm-pyro led to activation of the inflammasome, macrophage pyroptosis and ultimately attenuation of virulence. Depletion of caspase A, the zebrafish caspase-1 homolog, restored Lm-pyro virulence. Inflammasome activation specifically recruited macrophages to infection sites, whereas neutrophils were equally recruited to wild type and Lm-pyro infections. Similar to caspase A depletion, macrophage deficiency rescued Lm-pyro virulence to wild-type levels, while defective neutrophils had no specific effect. Neutrophils were, however, important for general clearance of L. monocytogenes, as both wild type and Lm-pyro were more virulent in larvae with defective neutrophils. This study characterizes a novel model for inflammasome studies in an intact host, establishes the importance of macrophages during inflammasome responses and adds importance to the role of neutrophils in controlling L. monocytogenes infections.
© 2015 John Wiley & Sons Ltd.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

