Pediatric laryngotracheal reconstruction with tissue-engineered cartilage in a rabbit model
- PMID: 26468093
- PMCID: PMC7941182
- DOI: 10.1002/lary.25676
Pediatric laryngotracheal reconstruction with tissue-engineered cartilage in a rabbit model
Abstract
Objectives/hypothesis: To develop an effective rabbit model of in vitro- and in vivo-derived tissue-engineered cartilage for laryngotracheal reconstruction (LTR).
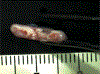
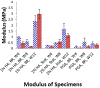
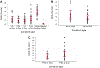
Study design: 1) Determination of the optimal scaffold 1% hyaluronic acid (HA), 2% HA, and polyglycolic acid (PGA) and in vitro culture time course using a pilot study of 4 by 4-mm in vitro-derived constructs analyzed on a static culture versus zero-gravity bioreactor for 4, 8, and 12 weeks, with determination of compressive modulus and histology as outcome measures. 2) Three-stage survival rabbit experiment utilizing autologous auricular chondrocytes seeded in scaffolds, either 1% HA or PGA. The constructs were cultured for the determined in vitro time period and then cultured in vivo for 12 weeks. Fifteen LTRs were performed using HA cartilage constructs, and one was performed with a PGA construct. All remaining specimens and the final reconstructed larynx underwent mechanical testing, histology, and glycosaminoglycan (GAG) content determination, and then were compared to cricoid control specimens (n = 13) and control LTR using autologous thyroid cartilage (n = 18).
Methods: 1) One rabbit underwent an auricular punch biopsy, and its chondrocytes were isolated and expanded and then encapsulated in eight 4 by 4-mm discs of 1% HA, 2% HA, PGA either in rotary bioreactor or static culture for 4, 8, and 12 weeks, respectively, with determination of compressive modulus, GAG content, and histology. 2) Sixteen rabbits underwent ear punch biopsy; chondrocytes were isolated and expanded. The cells were seeded in 13 by 5 by 2.25-mm UV photopolymerized 1% HA (w/w) or calcium alginate encapsulated synthetic PGA (13 × 5 × 2 mm); the constructs were then incubated in vitro for 12 weeks (the optimal time period determined above in paragraph 1) on a shaker. One HA and one PGA construct from each animal was tested mechanically and histologically, and the remaining eight (4 HA and 4 PGA) were implanted in the neck. After 12 weeks in vivo, the most optimal-appearing HA construct was used as a graft for LTR in 15 rabbits and PGA in one rabbit. The seven remaining specimens underwent hematoxylin and eosin, Safranin O, GAG content determination, and flexural modulus testing. At 12 weeks postoperative, the animals were euthanized and underwent endoscopy. The larynges underwent mechanical and histological testing. All animals that died underwent postmortem examination, including gross and microhistological analysis of the reconstructed airway.
Results: Thirteen of the 15 rabbits that underwent LTR with HA in vitro- and in vivo-derived tissue-engineered cartilage constructs survived. The 1% HA specimens had the highest modulus and GAG after 12 weeks in vitro. The HA constructs became well integrated in the airway, supported respiration for the 12 weeks, and were histologically and mechanically similar to autologous cartilage.
Conclusions: The engineering of in vitro- and in vivo-derived cartilage with HA is a novel approach for laryngotracheal reconstruction. The data suggests that the in vitro- and in vivo-derived tissue-engineered approaches may offer a promising alternative to current strategies used in pediatric airway reconstruction, as well as other head and neck applications.
Level of evidence: NA. Laryngoscope, 126:S5-S21, 2016.
Keywords: Polyglycolic acid (PGA); compressive modulus; flexural modulus; glycosaminoglycan (GAG); hyaluronic acid (HA).
© 2015 The American Laryngological, Rhinological and Otological Society, Inc.
Figures









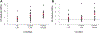






References
-
- Cotton RT. The problem of pediatric laryngotracheal stenosis: a clinical and experimental study on the efficacy of autologous cartilage grafts placed between the vertically divided halves of the posterior lamina of the cricoid cartilage. Laryngoscope 1991;101(suppl 56):1–34. - PubMed
-
- Hartnick CJ, Hartley BE, Lacy PD, et al. Surgery for pediatric subglottic stenosis-disease specific outcomes. Ann Otol Rhinol Laryngol 2001;110: 1109–1113. - PubMed
-
- Rizzi M, Thorne MC, Zur KB, Jacobs IN. Laryngotracheal reconstruction using posterior costal cartilage grafts: outcomes at a single institution. Otolaryngol Head Neck Surg 2009;140:348–353. - PubMed
-
- Jacobs IN, Podrebarac P, Boden SD, Chen M. Graft-healing in laryngotracheal reconstruction: an experimental rabbit model. Ann Otol Laryngol Rhinol 1999;108:599–605. - PubMed
-
- Langer R, Vacanti JP. Tissue-engineering. Science 1993;260:920–926. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

