Enhanced Functional Activity of the Cannabinoid Type-1 Receptor Mediates Adolescent Behavior
- PMID: 26468198
- PMCID: PMC4604232
- DOI: 10.1523/JNEUROSCI.1937-15.2015
Enhanced Functional Activity of the Cannabinoid Type-1 Receptor Mediates Adolescent Behavior
Abstract
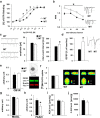
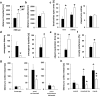
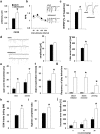
Adolescence is characterized by drastic behavioral adaptations and comprises a particularly vulnerable period for the emergence of various psychiatric disorders. Growing evidence reveals that the pathophysiology of these disorders might derive from aberrations of normal neurodevelopmental changes in the adolescent brain. Understanding the molecular underpinnings of adolescent behavior is therefore critical for understanding the origin of psychopathology, but the molecular mechanisms that trigger adolescent behavior are unknown. Here, we hypothesize that the cannabinoid type-1 receptor (CB1R) may play a critical role in mediating adolescent behavior because enhanced endocannabinoid (eCB) signaling has been suggested to occur transiently during adolescence. To study enhanced CB1R signaling, we introduced a missense mutation (F238L) into the rat Cnr1 gene that encodes for the CB1R. According to our hypothesis, rats with the F238L mutation (Cnr1(F238L)) should sustain features of adolescent behavior into adulthood. Gain of function of the mutated receptor was demonstrated by in silico modeling and was verified functionally in a series of biochemical and electrophysiological experiments. Mutant rats exhibit an adolescent-like phenotype during adulthood compared with wild-type littermates, with typical high risk/novelty seeking, increased peer interaction, enhanced impulsivity, and augmented reward sensitivity for drug and nondrug reward. Partial inhibition of CB1R activity in Cnr1(F238L) mutant rats normalized behavior and led to a wild-type phenotype. We conclude that the activity state and functionality of the CB1R is critical for mediating adolescent behavior. These findings implicate the eCB system as an important research target for the neuropathology of adolescent-onset mental health disorders.
Significance statement: We present the first rodent model with a gain-of-function mutation in the cannabinoid type-1 receptor (CB1R). Adult mutant rats exhibit an adolescent-like phenotype with typical high risk seeking, impulsivity, and augmented drug and nondrug reward sensitivity. Adolescence is a critical period for suboptimal behavioral choices and the emergence of neuropsychiatric disorders. Understanding the basis of these disorders therefore requires a comprehensive knowledge of how adolescent neurodevelopment triggers behavioral reactions. Our behavioral observations in adult mutant rats, together with reports on enhanced adolescent CB1R signaling, suggest a pivotal role for the CB1R in an adolescent brain as an important molecular mediator of adolescent behavior. These findings implicate the endocannabinoid system as a notable research target for adolescent-onset mental health disorders.
Keywords: CB1 receptor; ENU mutagenesis; adolescence; endocannabinoids; reward processing; risk seeking.
Copyright © 2015 the authors 0270-6474/15/3513976-14$15.00/0.
Figures




References
-
- Ahn K, Johnson DS, Mileni M, Beidler D, Long JZ, McKinney MK, Weerapana E, Sadagopan N, Liimatta M, Smith SE, Lazerwith S, Stiff C, Kamtekar S, Bhattacharya K, Zhang Y, Swaney S, Van Becelaere K, Stevens RC, Cravatt BF. Discovery and characterization of a highly selective FAAH inhibitor that reduces inflammatory pain. Chem Biol. 2009;16:411–420. doi: 10.1016/j.chembiol.2009.02.013. - DOI - PMC - PubMed
-
- Ahuja S, Hornak V, Yan EC, Syrett N, Goncalves JA, Hirshfeld A, Ziliox M, Sakmar TP, Sheves M, Reeves PJ, Smith SO, Eilers M. Helix movement is coupled to displacement of the second extracellular loop in rhodopsin activation. Nat Struct Mol Biol. 2009;16:168–175. doi: 10.1038/nsmb.1549. - DOI - PMC - PubMed
-
- Anavi-Goffer S, Fleischer D, Hurst DP, Lynch DL, Barnett-Norris J, Shi S, Lewis DL, Mukhopadhyay S, Howlett AC, Reggio PH, Abood ME. Helix 8 Leu in the CB1 cannabinoid receptor contributes to selective signal transduction mechanisms. J Biol Chem. 2007;282:25100–25113. doi: 10.1074/jbc.M703388200. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
