AztD, a Periplasmic Zinc Metallochaperone to an ATP-binding Cassette (ABC) Transporter System in Paracoccus denitrificans
- PMID: 26468286
- PMCID: PMC4705993
- DOI: 10.1074/jbc.M115.684506
AztD, a Periplasmic Zinc Metallochaperone to an ATP-binding Cassette (ABC) Transporter System in Paracoccus denitrificans
Abstract
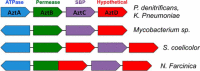
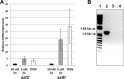
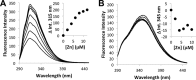
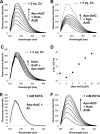
Bacterial ATP-binding cassette (ABC) transporters of transition metals are essential for acquisition of necessary elements from the environment. A large number of Gram-negative bacteria, including human pathogens, have a fourth conserved gene of unknown function adjacent to the canonical permease, ATPase, and solute-binding protein (SBP) genes of the AztABC zinc transporter system. To assess the function of this putative accessory factor (AztD) from Paracoccus denitrificans, we have analyzed its transcriptional regulation, metal binding properties, and interaction with the SBP (AztC). Transcription of the aztD gene is significantly up-regulated under conditions of zinc starvation. Recombinantly expressed AztD purifies with slightly substoichiometric zinc from the periplasm of Escherichia coli and is capable of binding up to three zinc ions with high affinity. Size exclusion chromatography and a simple intrinsic fluorescence assay were used to determine that AztD as isolated is able to transfer bound zinc nearly quantitatively to apo-AztC. Transfer occurs through a direct, associative mechanism that prevents loss of metal to the solvent. These results indicate that AztD is a zinc chaperone to AztC and likely functions to maintain zinc homeostasis through interaction with the AztABC system. This work extends our understanding of periplasmic zinc trafficking and the function of chaperones in this process.
Keywords: ABC transporter; chaperone; metal homeostasis; metalloprotein; zinc.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



References
-
- Coleman J. E. (1992) Zinc proteins: enzymes, storage proteins, transcription factors, and replication proteins. Annu. Rev. Biochem. 61, 897–946 - PubMed
-
- Hantke K. (2005) Bacterial zinc uptake and regulators. Curr. Opin. Microbiol. 8, 196–202 - PubMed
-
- Nies D. H. (2003) Efflux-mediated heavy metal resistance in prokaryotes. FEMS Microbiol. Rev. 27, 313–339 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

