Traditional serrated adenomas of the upper digestive tract
- PMID: 26468393
- PMCID: PMC4717432
- DOI: 10.1136/jclinpath-2015-203258
Traditional serrated adenomas of the upper digestive tract
Abstract
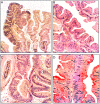
For many years, it was generally accepted that the vast majority of the colorectal carcinomas (CRCs) evolved from conventional adenomas, via the adenoma-carcinoma sequence. More recently, serrated colorectal polyps (hyperplastic polyps, sessile serrated polyps and traditional serrated adenomas (TSAs)) have emerged as an alternative pathway of colorectal carcinogenesis. It has been estimated that about 30% of the CRC progress via the serrated pathway. Recently, TSAs were also detected in the upper digestive tract. In this work, we review the literature on TSA in the oesophagus, the stomach, the duodenum, the pancreatic main duct and the gallbladder. The review indicated that 53.4% (n=39) out of the 73 TSA of the upper digestive tract now in record showed a simultaneously growing invasive carcinoma. As a corollary, TSAs of the upper digestive tract are aggressive adenomas that should be radically excised, either endoscopically or surgically, to rule out the possibility of a synchronously growing invasive adenocarcinoma or to prevent cancer progression. The present findings substantiate a TSA pathway of carcinogenesis in the upper digestive tract.
Keywords: GALL BLADDER CANCER; GASTRIC CANCER; OESOPHAGUS; PANCREATIC CANCER.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/
Figures




References
-
- O'Brien MJ, Gibbons D. The adenoma–carcinoma sequence in colorectal neoplasia. Surg Oncol Clin N Am 1996;5:513–22. - PubMed
-
- Snover DC, Jass JR, Fenoglio-Preiser C, et al. . Serrated polyps of the large intestine: a morphologic and molecular review of an evolving concept. Am J Surg Pathol 2005;124:380–91. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
