Induction of Broad-Based Immunity and Protective Efficacy by Self-amplifying mRNA Vaccines Encoding Influenza Virus Hemagglutinin
- PMID: 26468547
- PMCID: PMC4702536
- DOI: 10.1128/JVI.01786-15
Induction of Broad-Based Immunity and Protective Efficacy by Self-amplifying mRNA Vaccines Encoding Influenza Virus Hemagglutinin
Abstract
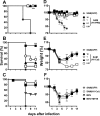
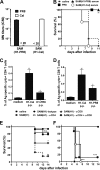
Seasonal influenza is a vaccine-preventable disease that remains a major health problem worldwide, especially in immunocompromised populations. The impact of influenza disease is even greater when strains drift, and influenza pandemics can result when animal-derived influenza virus strains combine with seasonal strains. In this study, we used the SAM technology and characterized the immunogenicity and efficacy of a self-amplifying mRNA expressing influenza virus hemagglutinin (HA) antigen [SAM(HA)] formulated with a novel oil-in-water cationic nanoemulsion. We demonstrated that SAM(HA) was immunogenic in ferrets and facilitated containment of viral replication in the upper respiratory tract of influenza virus-infected animals. In mice, SAM(HA) induced potent functional neutralizing antibody and cellular immune responses, characterized by HA-specific CD4 T helper 1 and CD8 cytotoxic T cells. Furthermore, mice immunized with SAM(HA) derived from the influenza A virus A/California/7/2009 (H1N1) strain (Cal) were protected from a lethal challenge with the heterologous mouse-adapted A/PR/8/1934 (H1N1) virus strain (PR8). Sera derived from SAM(H1-Cal)-immunized animals were not cross-reactive with the PR8 virus, whereas cross-reactivity was observed for HA-specific CD4 and CD8 T cells. Finally, depletion of T cells demonstrated that T-cell responses were essential in mediating heterologous protection. If the SAM vaccine platform proves safe, well tolerated, and effective in humans, the fully synthetic SAM vaccine technology could provide a rapid response platform to control pandemic influenza.
Importance: In this study, we describe protective immune responses in mice and ferrets after vaccination with a novel HA-based influenza vaccine. This novel type of vaccine elicits both humoral and cellular immune responses. Although vaccine-specific antibodies are the key players in mediating protection from homologous influenza virus infections, vaccine-specific T cells contribute to the control of heterologous infections. The rapid production capacity and the synthetic origin of the vaccine antigen make the SAM platform particularly exploitable in case of influenza pandemic.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures




References
-
- Tu W, Mao H, Zheng J, Liu Y, Chiu SS, Qin G, Chan PL, Lam KT, Guan J, Zhang L, Guan Y, Yuen KY, Peiris JS, Lau YL. 2010. Cytotoxic T lymphocytes established by seasonal human influenza cross-react against 2009 pandemic H1N1 influenza virus. J Virol 84:6527–6535. doi: 10.1128/JVI.00519-10. - DOI - PMC - PubMed
-
- Hillaire ML, van Trierum SE, Kreijtz JH, Bodewes R, Geelhoed-Mieras MM, Nieuwkoop NJ, Fouchier RA, Kuiken T, Osterhaus AD, Rimmelzwaan GF. 2011. Cross-protective immunity against influenza pH1N1 2009 viruses induced by seasonal influenza A (H3N2) virus is mediated by virus-specific T cells. J Gen Virol 92:2339–2349. doi: 10.1099/vir.0.033076-0. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

