Mammalian Lipopolysaccharide Receptors Incorporated into the Retroviral Envelope Augment Virus Transmission
- PMID: 26468748
- PMCID: PMC4795803
- DOI: 10.1016/j.chom.2015.09.005
Mammalian Lipopolysaccharide Receptors Incorporated into the Retroviral Envelope Augment Virus Transmission
Abstract
The orally transmitted retrovirus mouse mammary tumor virus (MMTV) requires the intestinal microbiota for persistence. Virion-associated lipopolysaccharide (LPS) activates Toll-like receptor 4 (TLR4), stimulating production of the immunosuppressive cytokine IL-10 and MMTV evasion of host immunity. However, the mechanisms by which MMTV associates with LPS remain unknown. We find that the viral envelope contains the mammalian LPS-binding factors CD14, TLR4, and MD-2, which, in conjunction with LPS-binding protein (LBP), bind LPS to the virus and augment transmission. MMTV isolated from infected mice lacking these LBPs cannot engage LPS or stimulate TLR4 and have a transmission defect. Furthermore, MMTV incorporation of a weak agonist LPS from Bacteroides, a prevalent LPS source in the gut, significantly enhances the ability of this LPS to stimulate TLR4, suggesting that MMTV intensifies these immunostimulatory properties. Thus, an orally transmitted retrovirus can capture, modify, and exploit mammalian receptors for bacterial ligands to ensure successful transmission.
Copyright © 2015 Elsevier Inc. All rights reserved.
Conflict of interest statement
None of the authors of this manuscript have a financial interest related to this work.
Figures

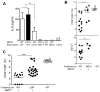

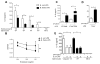
References
-
- Bubbers JE, Lilly F. Selective incorporation of H-2 antigenic determinants into Friend virus particles. Nature. 1977;266:458–459. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

