Resolvin D1 Reduces Emphysema and Chronic Inflammation
- PMID: 26468975
- PMCID: PMC4729265
- DOI: 10.1016/j.ajpath.2015.08.008
Resolvin D1 Reduces Emphysema and Chronic Inflammation
Abstract
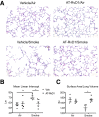
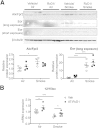
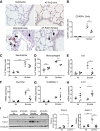
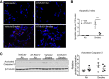
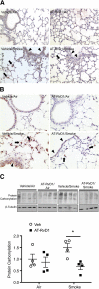
Chronic obstructive pulmonary disease is characterized, in part, by chronic inflammation that persists even after smoking cessation, suggesting that a failure to resolve inflammation plays an important role in the pathogenesis of the disease. It is widely recognized that the resolution of inflammation is an active process, governed by specialized proresolving lipid mediators, including lipoxins, resolvins, maresins, and protectins. Here, we report that proresolving signaling and metabolic pathways are disrupted in lung tissue from patients with chronic obstructive pulmonary disease, suggesting that supplementation with proresolving lipid mediators might reduce the development of emphysema by controlling chronic inflammation. Groups of mice were exposed long-term to cigarette smoke and treated with the proresolving mediator resolvin D1. Resolvin D1 was associated with a reduced development of cigarette smoke-induced emphysema and airspace enlargement, with concurrent reductions in inflammation, oxidative stress, and cell death. Interestingly, resolvin D1 did not promote the differentiation of M2 macrophages and did not promote tissue fibrosis. Taken together, our results suggest that cigarette smoking disrupts endogenous proresolving pathways and that supplementation with specialized proresolving lipid mediators is an important therapeutic strategy in chronic lung disease, especially if endogenous specialized proresolving lipid mediator signaling is impaired.
Copyright © 2015 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Yoshida T., Tuder R.M. Pathobiology of cigarette smoke-induced chronic obstructive pulmonary disease. Physiol Rev. 2007;87:1047–1082. - PubMed
-
- Salvi S., Barnes P.J. Is exposure to biomass smoke the biggest risk factor for COPD globally? Chest. 2010;138:3–6. - PubMed
-
- Barnes P.J. New therapies for chronic obstructive pulmonary disease. Med Princ Pract. 2010;19:330–338. - PubMed
-
- Barnes P.J. Mediators of chronic obstructive pulmonary disease. Pharmacol Rev. 2004;56:515–548. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32HL066988/HL/NHLBI NIH HHS/United States
- R01 GM038765/GM/NIGMS NIH HHS/United States
- P30 ES001247/ES/NIEHS NIH HHS/United States
- UL1 TR000042/TR/NCATS NIH HHS/United States
- GM038765/GM/NIGMS NIH HHS/United States
- P30ES001247/ES/NIEHS NIH HHS/United States
- R37 GM038765/GM/NIGMS NIH HHS/United States
- UL1TR000042/TR/NCATS NIH HHS/United States
- R01 HL120908/HL/NHLBI NIH HHS/United States
- T32 ES007026/ES/NIEHS NIH HHS/United States
- R01HL120908/HL/NHLBI NIH HHS/United States
- T32 HL066988/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

