Glia-derived neurons are required for sex-specific learning in C. elegans
- PMID: 26469050
- PMCID: PMC4650210
- DOI: 10.1038/nature15700
Glia-derived neurons are required for sex-specific learning in C. elegans
Abstract
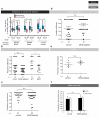
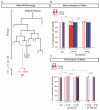
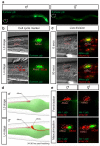
Sex differences in behaviour extend to cognitive-like processes such as learning, but the underlying dimorphisms in neural circuit development and organization that generate these behavioural differences are largely unknown. Here we define at the single-cell level-from development, through neural circuit connectivity, to function-the neural basis of a sex-specific learning in the nematode Caenorhabditis elegans. We show that sexual conditioning, a form of associative learning, requires a pair of male-specific interneurons whose progenitors are fully differentiated glia. These neurons are generated during sexual maturation and incorporated into pre-exisiting sex-shared circuits to couple chemotactic responses to reproductive priorities. Our findings reveal a general role for glia as neural progenitors across metazoan taxa and demonstrate that the addition of sex-specific neuron types to brain circuits during sexual maturation is an important mechanism for the generation of sexually dimorphic plasticity in learning.
Figures








References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

