Completeness of Follow-Up Determines Validity of Study Findings: Results of a Prospective Repeated Measures Cohort Study
- PMID: 26469346
- PMCID: PMC4607456
- DOI: 10.1371/journal.pone.0140817
Completeness of Follow-Up Determines Validity of Study Findings: Results of a Prospective Repeated Measures Cohort Study
Abstract
Background: Current reporting guidelines do not call for standardised declaration of follow-up completeness, although study validity depends on the representativeness of measured outcomes. The Follow-Up Index (FUI) describes follow-up completeness at a given study end date as ratio between the investigated and the potential follow-up period. The association between FUI and the accuracy of survival-estimates was investigated.
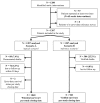
Methods: FUI and Kaplan-Meier estimates were calculated twice for 1207 consecutive patients undergoing aortic repair during an 11-year period: in a scenario A the population's clinical routine follow-up data (available from a prospective registry) was analysed conventionally. For the control scenario B, an independent survey was completed at the predefined study end. To determine the relation between FUI and the accuracy of study findings, discrepancies between scenarios regarding FUI, follow-up duration and cumulative survival-estimates were evaluated using multivariate analyses.
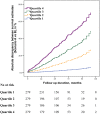
Results: Scenario A noted 89 deaths (7.4%) during a mean considered follow-up of 30±28months. Scenario B, although analysing the same study period, detected 304 deaths (25.2%, P<0.001) as it scrutinized the complete follow-up period (49±32months). FUI (0.57±0.35 versus 1.00±0, P<0.001) and cumulative survival estimates (78.7% versus 50.7%, P<0.001) differed significantly between scenarios, suggesting that incomplete follow-up information led to underestimation of mortality. Degree of follow-up completeness (i.e. FUI-quartiles and FUI-intervals) correlated directly with accuracy of study findings: underestimation of long-term mortality increased almost linearly by 30% with every 0.1 drop in FUI (adjusted HR 1.30; 95%-CI 1.24;1.36, P<0.001).
Conclusion: Follow-up completeness is a pre-requisite for reliable outcome assessment and should be declared systematically. FUI represents a simple measure suited as reporting standard. Evidence lacking such information must be challenged as potentially flawed by selection bias.
Conflict of interest statement
Figures


References
-
- Clark TG, Altman DG, De Stavola BL. Quantification of the completeness of follow-up. Lancet. 2002; 359(9314): 1309–10. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

