Liquid exfoliation of solvent-stabilized few-layer black phosphorus for applications beyond electronics
- PMID: 26469634
- PMCID: PMC4634220
- DOI: 10.1038/ncomms9563
Liquid exfoliation of solvent-stabilized few-layer black phosphorus for applications beyond electronics
Abstract
Few-layer black phosphorus (BP) is a new two-dimensional material which is of great interest for applications, mainly in electronics. However, its lack of environmental stability severely limits its synthesis and processing. Here we demonstrate that high-quality, few-layer BP nanosheets, with controllable size and observable photoluminescence, can be produced in large quantities by liquid phase exfoliation under ambient conditions in solvents such as N-cyclohexyl-2-pyrrolidone (CHP). Nanosheets are surprisingly stable in CHP, probably due to the solvation shell protecting the nanosheets from reacting with water or oxygen. Experiments, supported by simulations, show reactions to occur only at the nanosheet edge, with the rate and extent of the reaction dependent on the water/oxygen content. We demonstrate that liquid-exfoliated BP nanosheets are potentially useful in a range of applications from ultrafast saturable absorbers to gas sensors to fillers for composite reinforcement.
Figures

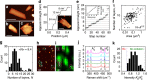
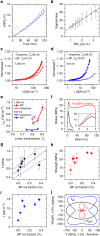
 behaviour. (g) Histogram of number of monolayers per nanosheet (sample size=126). The mean number of layers is determined as 9.4±1.3 nm (where the error is due to the uncertainty in the step height analysis and the s.e. of the distribution). (h,i) Large area AFM image (h, scale bar, 1 μm) and Raman A1g intensity map (i, excitation wavelength 633 nm) of the same sample region. (j) Raman spectra (normalized to Ag2) of the nanosheets indicated in h and i (the numbers labelling the spectra in j correspond to the nanosheets marked by numbers in h and i). (k) Histogram of the intensity ratio of the Ag1/Ag2 modes obtained from the analysis of 120 baseline-corrected spectra acquired over an area of 25 × 25 μm2 (sample size=120) The absence of spectra with an intensity ratio <0.6 strongly suggests that no basal plane oxidation has occurred.
behaviour. (g) Histogram of number of monolayers per nanosheet (sample size=126). The mean number of layers is determined as 9.4±1.3 nm (where the error is due to the uncertainty in the step height analysis and the s.e. of the distribution). (h,i) Large area AFM image (h, scale bar, 1 μm) and Raman A1g intensity map (i, excitation wavelength 633 nm) of the same sample region. (j) Raman spectra (normalized to Ag2) of the nanosheets indicated in h and i (the numbers labelling the spectra in j correspond to the nanosheets marked by numbers in h and i). (k) Histogram of the intensity ratio of the Ag1/Ag2 modes obtained from the analysis of 120 baseline-corrected spectra acquired over an area of 25 × 25 μm2 (sample size=120) The absence of spectra with an intensity ratio <0.6 strongly suggests that no basal plane oxidation has occurred.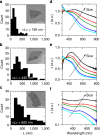

References
-
- Geim A. K. Graphene: status and prospects. Science 324, 1530–1534 (2009). - PubMed
-
- Novoselov K. S. et al.. A roadmap for graphene. Nature 490, 192–200 (2012). - PubMed
-
- Ferrari A. C. et al.. Science and technology roadmap for graphene, related two-dimensional crystals, and hybrid systems. Nanoscale 7, 4598–4810 (2015). - PubMed
-
- Chhowalla M. et al.. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 5, 263–275 (2013). - PubMed
-
- Osada M. & Sasaki T. Exfoliated oxide nanosheets: new solution to nanoelectronics. J. Mater. Chem. 19, 2503–2511 (2009).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

