HMGB1 induction of clusterin creates a chemoresistant niche in human prostate tumor cells
- PMID: 26469759
- PMCID: PMC4606829
- DOI: 10.1038/srep15085
HMGB1 induction of clusterin creates a chemoresistant niche in human prostate tumor cells
Abstract
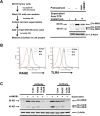
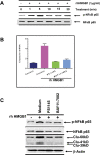
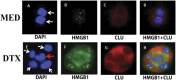
Development of chemoresistance, especially to docetaxel (DTX), is the primary barrier to the cure of castration-resistant prostate cancer but its mechanism is obscure. Here, we report a seminal crosstalk between dying and residual live tumor cells during treatment with DTX that can result in outgrowth of a chemoresistant population. Survival was due to the induction of secretory/cytoplasmic clusterin (sCLU), which is a potent anti-apoptotic protein known to bind and sequester Bax from mitochondria, to prevent caspase 3 activation. sCLU induction in live cells depended on HMGB1 release from dying cells. Supernatants from DTX-treated DU145 tumor cells, which were shown to contain HMGB1, effectively induced sCLU from newly-plated DU145 tumor cells and protected them from DTX toxicity. Addition of anti-HMBG1 to the supernatant or pretreatment of newly-plated DU145 tumor cells with anti-TLR4 or anti-RAGE markedly abrogated sCLU induction and protective effect of the supernatant. Mechanistically, HMGB1 activated NFκB to promote sCLU gene expression and prevented the translocation of activated Bax to mitochondria to block cell death. Importantly, multiple currently-used chemotherapeutic drugs could release HMGB1 from tumor cells. These results suggest that acquisition of chemoresistance may involve the HMGB1/TLR4-RAGE/sCLU pathway triggered by dying cells to provide survival advantage to remnant live tumor cells.
Figures



References
-
- Borst P., Jonkers J. & Rottenberg S. What makes tumors multidrug resistant? Cell cycle 6, 2782–2787 (2007). - PubMed
-
- Patterson S. G. et al. Novel role of Stat1 in the development of docetaxel resistance in prostate tumor cells. Oncogene 25, 6113–6122 (2006). - PubMed
-
- Steinberg J. et al. Intracellular levels of SGP-2 (Clusterin) correlate with tumor grade in prostate cancer. Clin Cancer Res 3, 1707–1711 (1997). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

