Dengue-3 Virus Entry into Vero Cells: Role of Clathrin-Mediated Endocytosis in the Outcome of Infection
- PMID: 26469784
- PMCID: PMC4607419
- DOI: 10.1371/journal.pone.0140824
Dengue-3 Virus Entry into Vero Cells: Role of Clathrin-Mediated Endocytosis in the Outcome of Infection
Abstract
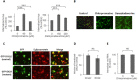
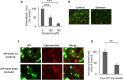
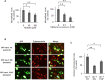
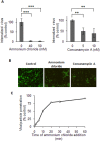
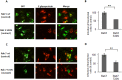
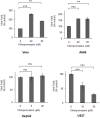
The endocytic uptake and intracellular trafficking for penetration of DENV-3 strain H-87 into Vero cells was analyzed by using several biochemical inhibitors and dominant negative mutants of cellular proteins. The results presented show that the infective entry of DENV-3 into Vero cells occurs through a non-classical endocytosis pathway dependent on low pH and dynamin, but non-mediated by clathrin. After uptake, DENV-3 transits through early endosomes to reach Rab 7-regulated late endosomes, and according with the half-time for ammonium chloride resistance viral nucleocapsid is released into the cytosol approximately at 12 min post-infection. Furthermore, the influence of the clathrin pathway in DENV-3 infective entry in other mammalian cell lines of human origin, such as A549, HepG2 and U937 cells, was evaluated demonstrating that variable entry pathways are employed depending on the host cell. Results show for the first time the simultaneous coexistence of infective and non -infective routes for DENV entry into the host cell, depending on the usage of clathrin-mediated endocytosis.
Conflict of interest statement
Figures
References
-
- Halstead SB. Dengue. Lancet. 2007; 370: 1644–1652. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

