Mutation of ataxia-telangiectasia mutated is associated with dysfunctional glutathione homeostasis in cerebellar astroglia
- PMID: 26469940
- PMCID: PMC5580048
- DOI: 10.1002/glia.22925
Mutation of ataxia-telangiectasia mutated is associated with dysfunctional glutathione homeostasis in cerebellar astroglia
Abstract
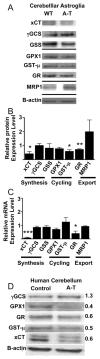
Astroglial dysfunction plays an important role in neurodegenerative diseases otherwise attributed to neuronal loss of function. Here we focus on the role of astroglia in ataxia-telangiectasia (A-T), a disease caused by mutations in the ataxia-telangiectasia mutated (ATM) gene. A hallmark of A-T pathology is progressive loss of cerebellar neurons, but the mechanisms that impact neuronal survival are unclear. We now provide a possible mechanism by which A-T astroglia affect the survival of cerebellar neurons. As astroglial functions are difficult to study in an in vivo setting, particularly in the cerebellum where these cells are intertwined with the far more numerous neurons, we conducted in vitro coculture experiments that allow for the generation and pharmacological manipulation of purified cell populations. Our analyses revealed that cerebellar astroglia isolated from Atm mutant mice show decreased expression of the cystine/glutamate exchanger subunit xCT, glutathione (GSH) reductase, and glutathione-S-transferase. We also found decreased levels of intercellular and secreted GSH in A-T astroglia. Metabolic labeling of l-cystine, the major precursor for GSH, revealed that a key component of the defect in A-T astroglia is an impaired ability to import this rate-limiting precursor for the production of GSH. This impairment resulted in suboptimal extracellular GSH supply, which in turn impaired survival of cerebellar neurons. We show that by circumventing the xCT-dependent import of L-cystine through addition of N-acetyl-L-cysteine (NAC) as an alternative cysteine source, we were able to restore GSH levels in A-T mutant astroglia providing a possible future avenue for targeted therapeutic intervention.
Keywords: astroglia; ataxia-telangiectasia; ataxia-telangiectasia mutated; cerebellum; glutathione; neuronal survival; xCT.
© 2015 Wiley Periodicals, Inc.
Figures





References
-
- Al-Ali H, Blackmore M, Bixby JL, Lemmon VP. High content screening with primary neurons. In: Sittampalam GS, Gal-Edd N, Arkin M, Auld D, Austin C, Bejcek B, Glicksman M, Inglese J, Lemmon V, Li Z, McGee J, McManus O, Minor L, Napper A, Peltier JM, Riss T, Trask OJ Jr, Weidner J, editors. Assay Guidance Manual e-book Eli Lilly & Company and the National Center for Advancing Translational Sciences. Bethesda, MD: 2004. - PubMed
-
- Albrecht P, Lewerenz J, Dittmer S, Noack R, Maher P, Methner A. Mechanisms of oxidative glutamate toxicity: The glutamate/cystine antiporter system xc- as a neuroprotective drug target. CNS Neurol Disord Drug Targets. 2010;9:373–382. - PubMed
-
- Ambrose M, Goldstine JV, Gatti RA. Intrinsic mitochondrial dysfunction in ATM-deficient lymphoblastoid cells. Hum Mol Genet. 2007;16:2154–2164. - PubMed
-
- Amromin GD, Boder E, Teplitz R. Ataxia-telangiectasia with a 32 year survival. A clinicopathological report. J Neuropathol Exp Neurol. 1979;38:621–643. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

