Antigenically Modified Human Pluripotent Stem Cells Generate Antigen-Presenting Dendritic Cells
- PMID: 26471005
- PMCID: PMC4608011
- DOI: 10.1038/srep15262
Antigenically Modified Human Pluripotent Stem Cells Generate Antigen-Presenting Dendritic Cells
Abstract
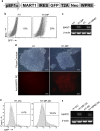
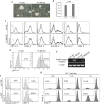
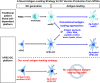
Human pluripotent stem cells (hPSCs) provide a promising platform to produce dendritic cell (DC) vaccine. To streamline the production process, we investigated a unique antigen-loading strategy that suits this novel platform. Specifically, we stably modified hPSCs using tumour antigen genes in the form of a full-length tumour antigen gene or an artificial tumour antigen epitope-coding minigene. Such antigenically modified hPSCs were able to differentiate into tumour antigen-presenting DCs. Without conventional antigen-loading, DCs derived from the minigene-modified hPSCs were ready to prime a tumour antigen-specific T cell response and further expand these specific T cells in restimulation processes. These expanded tumour antigen-specific T cells were potent effectors with central memory or effector memory phenotype. Thus, we demonstrated that immunocompetent tumour antigen-loaded DCs can be directly generated from antigenically modified hPSCs. Using such strategy, we can completely eliminate the conventional antigen-loading step and significantly simplify the production of DC vaccine from hPSCs.
Figures




Similar articles
-
Single-step antigen loading and activation of dendritic cells by mRNA electroporation for the purpose of therapeutic vaccination in melanoma patients.Clin Cancer Res. 2009 May 15;15(10):3366-75. doi: 10.1158/1078-0432.CCR-08-2982. Epub 2009 May 5. Clin Cancer Res. 2009. PMID: 19417017
-
Modification of human embryonic stem cell-derived dendritic cells with mRNA for efficient antigen presentation and enhanced potency.Regen Med. 2011 May;6(3):303-18. doi: 10.2217/rme.11.19. Regen Med. 2011. PMID: 21548736
-
Vaccination of metastatic colorectal cancer patients with matured dendritic cells loaded with multiple major histocompatibility complex class I peptides.J Immunother. 2007 Oct;30(7):762-72. doi: 10.1097/CJI.0b013e318133451c. J Immunother. 2007. PMID: 17893568 Clinical Trial.
-
Genetically engineered dendritic cell-based cancer vaccines (review).Int J Oncol. 2001 Mar;18(3):475-8. Int J Oncol. 2001. PMID: 11179474 Review.
-
TAA/ecdCD40L adenoviral prime-protein boost vaccine for cancer and infectious diseases.Cancer Gene Ther. 2013 Feb;20(2):65-9. doi: 10.1038/cgt.2012.87. Epub 2012 Dec 14. Cancer Gene Ther. 2013. PMID: 23238593 Review.
Cited by
-
Dendritic cell vaccines: A review of recent developments and their potential pediatric application.Hum Vaccin Immunother. 2016 Sep;12(9):2232-9. doi: 10.1080/21645515.2016.1179844. Epub 2016 May 31. Hum Vaccin Immunother. 2016. PMID: 27245943 Free PMC article. Review.
-
Dendritic cell-based immunotherapy.Cell Res. 2017 Jan;27(1):74-95. doi: 10.1038/cr.2016.157. Epub 2016 Dec 27. Cell Res. 2017. PMID: 28025976 Free PMC article. Review.
-
Manipulation of Regulatory Dendritic Cells for Induction Transplantation Tolerance.Front Immunol. 2020 Oct 14;11:582658. doi: 10.3389/fimmu.2020.582658. eCollection 2020. Front Immunol. 2020. PMID: 33162996 Free PMC article. Review.
-
Dendritic Cell Strategies for Eliciting Mutation-Derived Tumor Antigen Responses in Patients.Cancer J. 2017 Mar/Apr;23(2):131-137. doi: 10.1097/PPO.0000000000000251. Cancer J. 2017. PMID: 28410301 Free PMC article. Review.
-
Generation of "Off-the-Shelf" Natural Killer Cells from Peripheral Blood Cell-Derived Induced Pluripotent Stem Cells.Stem Cell Reports. 2017 Dec 12;9(6):1796-1812. doi: 10.1016/j.stemcr.2017.10.020. Epub 2017 Nov 22. Stem Cell Reports. 2017. PMID: 29173894 Free PMC article.
References
-
- Nicolette C. A. et al. Dendritic cells for active immunotherapy: optimizing design and manufacture in order to develop commercially and clinically viable products. Vaccine 25 Suppl 2, B47–60 (2007). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

