A Familial C3GN Secondary to Defective C3 Regulation by Complement Receptor 1 and Complement Factor H
- PMID: 26471127
- PMCID: PMC4884110
- DOI: 10.1681/ASN.2015040348
A Familial C3GN Secondary to Defective C3 Regulation by Complement Receptor 1 and Complement Factor H
Abstract
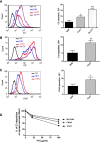
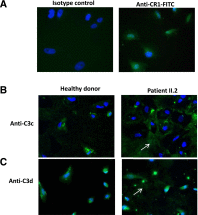
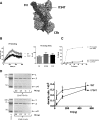
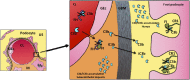
C3 glomerulopathy is a recently described form of CKD. C3GN is a subtype of C3 glomerulopathy characterized by predominant C3 deposits in the glomeruli and is commonly the result of acquired or genetic abnormalities in the alternative pathway (AP) of the complement system. We identified and characterized the first mutation of the C3 gene (p. I734T) in two related individuals diagnosed with C3GN. Immunofluorescence and electron microscopy studies showed C3 deposits in the subendothelial space, associated with unusual deposits located near the complement receptor 1 (CR1)-expressing podocytes. In vitro, this C3 mutation exhibited decreased binding to CR1, resulting in less CR1-dependent cleavage of C3b by factor 1. Both patients had normal plasma C3 levels, and the mutant C3 interacted with factor B comparably to wild-type (WT) C3 to form a C3 convertase. Binding of mutant C3 to factor H was normal, but mutant C3 was less efficiently cleaved by factor I in the presence of factor H, leading to enhanced C3 fragment deposition on glomerular cells. In conclusion, our results reveal that a CR1 functional deficiency is a mechanism of intraglomerular AP dysregulation and could influence the localization of the glomerular C3 deposits.
Keywords: C; glomerular disease; immunology and pathology.
Copyright © 2016 by the American Society of Nephrology.
Figures





References
-
- Fakhouri F, Frémeaux-Bacchi V, Noël LH, Cook HT, Pickering MC: C3 glomerulopathy: a new classification. Nat Rev Nephrol 6: 494–499, 2010 - PubMed
-
- Hou J, Markowitz GS, Bomback AS, Appel GB, Herlitz LC, Barry Stokes M, D’Agati VD: Toward a working definition of C3 glomerulopathy by immunofluorescence. Kidney Int 85: 450–456, 2014 - PubMed
-
- Pickering MC, D’Agati VD, Nester CM, Smith RJ, Haas M, Appel GB, Alpers CE, Bajema IM, Bedrosian C, Braun M, Doyle M, Fakhouri F, Fervenza FC, Fogo AB, Frémeaux-Bacchi V, Gale DP, Goicoechea de Jorge E, Griffin G, Harris CL, Holers VM, Johnson S, Lavin PJ, Medjeral-Thomas N, Paul Morgan B, Nast CC, Noel LH, Peters DK, Rodríguez de Córdoba S, Servais A, Sethi S, Song WC, Tamburini P, Thurman JM, Zavros M, Cook HT: C3 glomerulopathy: consensus report. Kidney Int 84: 1079–1089, 2013 - PMC - PubMed
-
- Sethi S, Fervenza FC: Membranoproliferative glomerulonephritis--a new look at an old entity. N Engl J Med 366: 1119–1131, 2012 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

