Endothelial Krüppel-Like Factor 4 Mediates the Protective Effect of Statins against Ischemic AKI
- PMID: 26471129
- PMCID: PMC4849832
- DOI: 10.1681/ASN.2015040460
Endothelial Krüppel-Like Factor 4 Mediates the Protective Effect of Statins against Ischemic AKI
Abstract
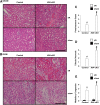
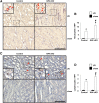
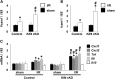
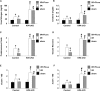
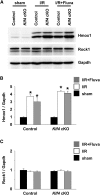
Endothelial cells participate in the pathophysiology of ischemic AKI by increasing the expression of cell adhesion molecules and by recruiting inflammatory cells. We previously showed that endothelial Krüppel-like factor 4 (Klf4) regulates vascular cell adhesion molecule 1 (Vcam1) expression and neointimal formation after carotid injury. In this study, we determined whether endothelial Klf4 is involved in ischemic AKI using endothelial Klf4 conditional knockout (Klf4 cKO) mice generated by breeding Tek-Cre mice and Klf4 floxed mice. Klf4 cKO mice were phenotypically normal before surgery. However, after renal ischemia-reperfusion injury, Klf4 cKO mice exhibited elevated serum levels of urea nitrogen and creatinine and aggravated renal histology compared with those of Klf4 floxed controls. Moreover, Klf4 cKO mice exhibited enhanced accumulation of neutrophils and lymphocytes and elevated expression of cell adhesion molecules, including Vcam1 and Icam1, in injured kidneys. Notably, statins ameliorated renal ischemia-reperfusion injury in control mice but not in Klf4 cKO mice. Mechanistic analyses in cultured endothelial cells revealed that statins increased KLF4 expression and that KLF4 mediated the suppressive effect of statins on TNF-α-induced VCAM1 expression by reducing NF-κB binding to the VCAM1 promoter. These results provide evidence that endothelial Klf4 is renoprotective and mediates statin-induced protection against ischemic AKI by regulating the expression of cell adhesion molecules and concomitant recruitment of inflammatory cells.
Keywords: acute renal failure; adhesion molecule; endothelial cells; ischemia-reperfusion; transcription factors.
Copyright © 2016 by the American Society of Nephrology.
Figures



References
-
- Rabb H, Mendiola CC, Saba SR, Dietz JR, Smith CW, Bonventre JV, Ramirez G: Antibodies to ICAM-1 protect kidneys in severe ischemic reperfusion injury. Biochem Biophys Res Commun 211: 67–73, 1995 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

