Enantioselective Synthesis of 3,5,6-Substituted Dihydropyranones and Dihydropyridinones using Isothiourea-Mediated Catalysis
- PMID: 26471245
- PMCID: PMC4755233
- DOI: 10.1002/asia.201500907
Enantioselective Synthesis of 3,5,6-Substituted Dihydropyranones and Dihydropyridinones using Isothiourea-Mediated Catalysis
Abstract
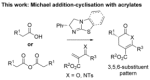
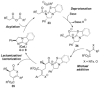
The scope of dihydropyranone and dihydropyridinone products accessible by isothiourea-catalyzed processes has been expanded and explored through the use of 2-N-tosyliminoacrylates and 2-aroylacrylates in a Michael addition-lactonization/lactamization cascade reaction. Notably, to ensure reproducibility it is essential to use homoanhydrides as ammonium enolate precursors with 2-aroyl acrylates, while carboxylic acids can be used with 2-N-tosyliminoacrylates, delivering a range of 3,5,6-substituted dihydropyranones and dihydropyridinones with high enantioselectivity (typically >90 % ee). The derivatization of the heterocyclic core of a 3,5,6-substituted dihydropyranone through hydrogenation is also reported.
Keywords: Michael addition; dihydropyranones; dihydropyridinones; enantioselective catalysis; isothioureas.
© 2015 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Figures





Similar articles
-
Multiple roles of aryloxide leaving groups in enantioselective annulations employing α,β-unsaturated acyl ammonium catalysis.Chem Sci. 2018 May 4;9(21):4909-4918. doi: 10.1039/c8sc01324a. eCollection 2018 Jun 7. Chem Sci. 2018. PMID: 29910944 Free PMC article.
-
Evaluating aryl esters as bench-stable C(1)-ammonium enolate precursors in catalytic, enantioselective Michael addition-lactonisations.Org Biomol Chem. 2019 May 15;17(19):4747-4752. doi: 10.1039/c9ob00703b. Org Biomol Chem. 2019. PMID: 30994694
-
Exploiting the Imidazolium Effect in Base-free Ammonium Enolate Generation: Synthetic and Mechanistic Studies.Angew Chem Int Ed Engl. 2016 Nov 7;55(46):14394-14399. doi: 10.1002/anie.201608046. Epub 2016 Oct 20. Angew Chem Int Ed Engl. 2016. PMID: 27762045
-
Generation and Reactivity of C(1)-Ammonium Enolates by Using Isothiourea Catalysis.Chemistry. 2021 Jan 21;27(5):1533-1555. doi: 10.1002/chem.202002059. Epub 2020 Nov 10. Chemistry. 2021. PMID: 32557875 Free PMC article. Review.
-
Base-free Enantioselective C(1)-Ammonium Enolate Catalysis Exploiting Aryloxides: A Synthetic and Mechanistic Study.Angew Chem Int Ed Engl. 2019 Oct 14;58(42):15111-15119. doi: 10.1002/anie.201908627. Epub 2019 Sep 12. Angew Chem Int Ed Engl. 2019. PMID: 31436380 Review.
Cited by
-
A Desilylative Approach to Alkyl Substituted C(1)-Ammonium Enolates: Application in Enantioselective [2+2] Cycloadditions.Angew Chem Int Ed Engl. 2022 Sep 19;61(38):e202208800. doi: 10.1002/anie.202208800. Epub 2022 Aug 8. Angew Chem Int Ed Engl. 2022. PMID: 35833471 Free PMC article.
-
Tandem sequential catalytic enantioselective synthesis of highly-functionalised tetrahydroindolizine derivatives.Chem Sci. 2020 Mar 12;11(15):3885-3892. doi: 10.1039/d0sc00432d. Chem Sci. 2020. PMID: 34122857 Free PMC article.
-
A facile method for Rh-catalyzed decarbonylative ortho-C-H alkylation of (hetero)arenes with alkyl carboxylic acids.RSC Adv. 2021 Jun 2;11(32):19827-19831. doi: 10.1039/d1ra03992j. eCollection 2021 May 27. RSC Adv. 2021. PMID: 35479217 Free PMC article.
-
Catalytic enantioselective synthesis of 1,4-dihydropyridines via the addition of C(1)-ammonium enolates to pyridinium salts.Chem Sci. 2021 Aug 6;12(36):12001-12011. doi: 10.1039/d1sc03860e. eCollection 2021 Sep 22. Chem Sci. 2021. PMID: 34667566 Free PMC article.
-
Enantioselective Synthesis of α-Aryl-β2 -Amino-Esters by Cooperative Isothiourea and Brønsted Acid Catalysis.Angew Chem Int Ed Engl. 2021 May 17;60(21):11892-11900. doi: 10.1002/anie.202016220. Epub 2021 May 4. Angew Chem Int Ed Engl. 2021. PMID: 33646631 Free PMC article.
References
-
- None
-
- Zhao H., Neamati N., Hong H., Mazumder A., Wang S., Sunder S., Milne G. W. A., Pommier Y., T. R. Burke, Jr. , J. Med. Chem. 1997, 40, 242–249; - PubMed
-
- Seo E.-K., Wani M. C., Wall M. E., Navarro H., Mukherjee R., Farnsworth N. R., Kinghorn A. D., Phytochemistry 2000, 55, 35–42; - PubMed
-
- Nantermet P. G., Barrow J. C., Selnick H. G., Homnick C. F., Freidinger R. M., Chang R. S. L., O'Malley S. S., Reiss D. R., Broten T. P., Ransom R. W., Pettibone D. J., Olah T., Forray C., Bioorg. Med. Chem. Lett. 2000, 10, 1625–1628; - PubMed
-
- Goodman K. B., Cui H., Dowdell S. E., Gaitanopoulos D. E., Ivy R. L., Sehon C. A., Stavenger R. A., Wang G. Z., Viet A. Q., Xu W., Ye G., Semus S. F., Evans C., Fries H. E., Jolivette L. J., Kirkpatrick R. B., Dul E., Khandekar S. S., Yi T., Jung D. K., Wright L. L., Smith G. K., Behm D. J., Bentley R., Doe C. P., Hu E., Lee D., J. Med. Chem. 2007, 50, 6–9. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

