Analysis of non-peptidic compounds as potential malarial inhibitors against Plasmodial cysteine proteases via integrated virtual screening workflow
- PMID: 26471975
- PMCID: PMC5035544
- DOI: 10.1080/07391102.2015.1108231
Analysis of non-peptidic compounds as potential malarial inhibitors against Plasmodial cysteine proteases via integrated virtual screening workflow
Abstract
Falcipain-2 (FP-2) and falcipain-3 (FP-3), haemoglobin-degrading enzymes in Plasmodium falciparum, are validated drug targets for the development of effective inhibitors against malaria. However, no commercial drug-targeting falcipains has been developed despite their central role in the life cycle of the parasites. In this work, in silico approaches are used to identify key structural elements that control the binding and selectivity of a diverse set of non-peptidic compounds onto FP-2, FP-3 and homologues from other Plasmodium species as well as human cathepsins. Hotspot residues and the underlying non-covalent interactions, important for the binding of ligands, are identified by interaction fingerprint analysis between the proteases and 2-cyanopyridine derivatives (best hits). It is observed that the size and chemical type of substituent groups within 2-cyanopyridine derivatives determine the strength of protein-ligand interactions. This research presents novel results that can further be exploited in the structure-based molecular-guided design of more potent antimalarial drugs.
Keywords: docking; falcipains; homology modelling; malaria; molecular dynamics.
Figures
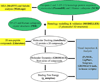






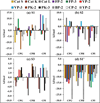

References
-
- Ashley E, McGready R, Proux S, Nosten F. Malaria. Travel Medicine and Infectious Disease. 2006;4(3-4):159–173. - PubMed
-
- Batra S, Sabnis YA, Rosenthal PJ, Avery MA. Structure-based approach to falcipain-2 inhibitors: synthesis and biological evaluation of 1,6,7-trisubstituted dihydroisoquinolines and isoquinolines. Bioorganic & Medicinal Chemistry. 2003;11(10):2293–2299. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
