TLR9-Targeted SiRNA Delivery In Vivo
- PMID: 26472451
- PMCID: PMC4816223
- DOI: 10.1007/978-1-4939-3112-5_15
TLR9-Targeted SiRNA Delivery In Vivo
Abstract
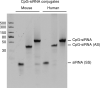
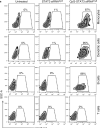
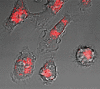
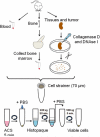
The SiRNA strategy is a potent and versatile method for modulating expression of any gene in various species for investigational or therapeutic purposes. Clinical translation of SiRNA-based approaches proved challenging, mainly due to the difficulty of targeted SiRNA delivery into cells of interest and the immunogenic side effects of oligonucleotide reagents. However, the intrinsic sensitivity of immune cells to nucleic acids can be utilized for the delivery of SiRNAs designed for the purpose of cancer immunotherapy. We have demonstrated that synthetic ligands for the intracellular receptor TLR9 can serve as targeting moiety for cell-specific delivery of SiRNAs. Chemically synthesized CpG-SiRNA conjugates are quickly internalized by TLR9-positive cells in the absence of transfection reagents, inducing target gene silencing. The CpG-SiRNA strategy allows for effective targeting of TLR9-positive cells in vivo after local or systemic administration of these oligonucleotides into mice.
Keywords: Cancer; CpG; Leukemia; Myeloid cells; Oligonucleotides; SiRNA; TLR9.
Figures


References
-
- Zimmermann TS, Lee AC, Akinc A, Bramlage B, Bumcrot D, Fedoruk MN, Harborth J, Heyes JA, Jeffs LB, John M, Judge AD, Lam K, McClintock K, Nechev LV, Palmer LR, Racie T, Rohl I, Seiffert S, Shanmugam S, Sood V, Soutschek J, Toudjarska I, Wheat AJ, Yaworski E, Zedalis W, Koteliansky V, Manoharan M, Vornlocher HP, MacLachlan I. RNAi-mediated gene silencing in non-human primates. Nature. 2006;441:111–114. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

