Effects of ceftiofur treatment on the susceptibility of commensal porcine E.coli--comparison between treated and untreated animals housed in the same stable
- PMID: 26472561
- PMCID: PMC4608134
- DOI: 10.1186/s12917-015-0578-3
Effects of ceftiofur treatment on the susceptibility of commensal porcine E.coli--comparison between treated and untreated animals housed in the same stable
Abstract
Background: Healthy farm animals have been found to act as a reservoir of extended-spectrum β-lactamase (ESBL)-producing Escherichia coli (E. coli). Therefore, the objective of the study was to determine the input of antimicrobial active ceftiofur metabolites in the stable via faeces and urine after intramuscular administration of the drug to pigs and the elucidation of the Escherichia coli ESBL resistance pattern of treated and untreated pigs housed in the same barn during therapy.
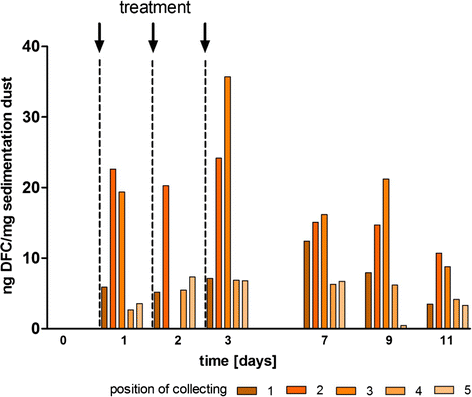
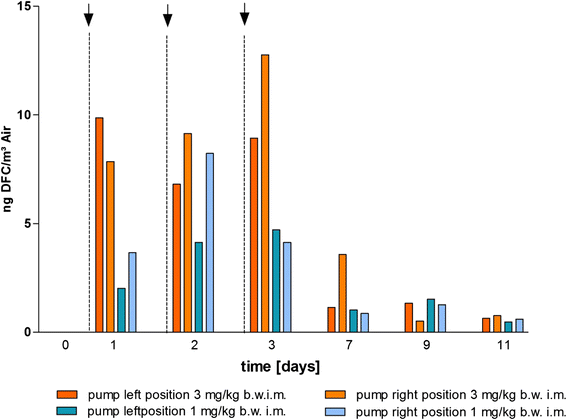
Methods: For determination of the minimal inhibitory concentration (MIC) the method of microdilutionaccording to the recommended procedure of the Clinical and Laboratory Standards Institute was used. Inaddition to that, a qualitative determination was performed by agar dilution. Unsusceptible E. coli speciesselected via agar dilution with cefotaxime were confirmed by MALDI-TOF and ESBL encoding genes wereidentified by PCR. The amounts of ceftiofur measured as desfuroylceftiofur (DFC) in the different probes (plasma, urine, faeces and dust) were analysed by UPLC-MS/MS.
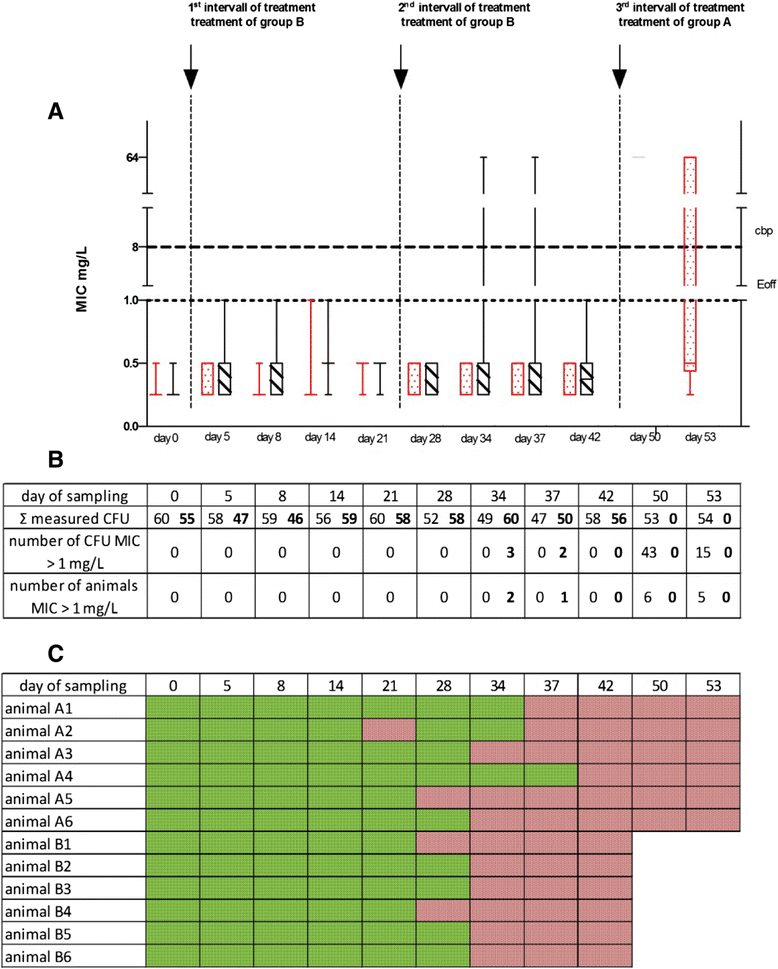
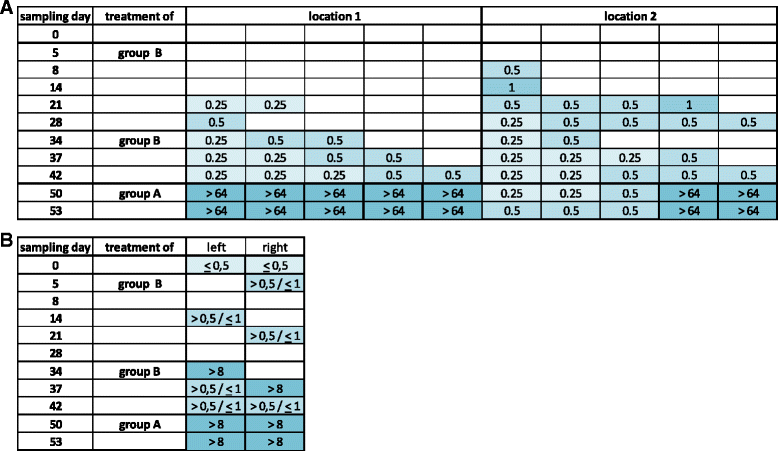
Results: In a first experiment two groups of pigs (6 animals per group) were housed in the same barn in two separated boxes. One group (group B) were treated with ceftiofur according to the licence (3 mg/kg administered intramuscularly (i.m.) on three consecutive days, day 1-3). During a second treatment period (day 29-31) an increased rate of ESBL resistant E. coli was detectable in these treated pigs and in the air of the stable. Moreover, the second group of animals (group A) formerly untreated but housed for the whole period in the same stable as the treated animals revealed increased resistance rates during their first treatment (day 45-47) with ceftiofur. In order to investigate the environmental input of ceftiofur during therapy and to simulate oral uptake of ceftiofur residues from the air of the stable a second set of experiments were performed. Pigs (6 animals) were treated with an interval of 2 weeks for 3 days with different doses of ceftiofur (3 mg/kg, 1 mg/kg and 0.3 mg/kg i.m.) as well as with 3 mg/kg per os) and the renal and biliary excretion of ceftiofur as its active metabolite were measured in comparison to the plasma levels. In addition to that, probes of the sedimentation dust and the air of the stable were analysed for drug residues.
Conclusion: The present study shows that treatment of several animals in a stable with ceftiofur influences the resistance pattern of intestinal Escherichia coli of the treated as well as untreated animals housed in the same stable. During therapy with the drug which was administered by injection according to the licence we detected nameable amounts of ceftiofur and its active metabolites in the dust and air of the stable.
Figures
Similar articles
-
Effects of intramuscularly administered enrofloxacin on the susceptibility of commensal intestinal Escherichia coli in pigs (sus scrofa domestica).BMC Vet Res. 2017 Dec 4;13(1):378. doi: 10.1186/s12917-017-1260-8. BMC Vet Res. 2017. PMID: 29202759 Free PMC article.
-
Characterization of extended-spectrum β-lactamase (ESBL)-producing Escherichia coli obtained from Danish pigs, pig farmers and their families from farms with high or no consumption of third- or fourth-generation cephalosporins.J Antimicrob Chemother. 2014 Oct;69(10):2650-7. doi: 10.1093/jac/dku180. Epub 2014 Jun 7. J Antimicrob Chemother. 2014. PMID: 24908045
-
Efficacy of ceftiofur hydrochloride for treatment of experimentally induced colibacillosis in neonatal swine.Am J Vet Res. 1990 Mar;51(3):349-53. Am J Vet Res. 1990. PMID: 2180350
-
Risk Factors for Antimicrobial Resistance in Escherichia coli in Pigs Receiving Oral Antimicrobial Treatment: A Systematic Review.Microb Drug Resist. 2017 Mar;23(2):194-205. doi: 10.1089/mdr.2015.0318. Epub 2016 Jun 1. Microb Drug Resist. 2017. PMID: 27249658
-
Cephalosporins in veterinary medicine - ceftiofur use in food animals.Curr Top Med Chem. 2002 Jul;2(7):717-31. doi: 10.2174/1568026023393679. Curr Top Med Chem. 2002. PMID: 12052187 Review.
Cited by
-
Characterization of Interactions between CTX-M-15 and Clavulanic Acid, Desfuroylceftiofur, Ceftiofur, Ampicillin, and Nitrocefin.Int J Mol Sci. 2022 May 7;23(9):5229. doi: 10.3390/ijms23095229. Int J Mol Sci. 2022. PMID: 35563620 Free PMC article.
-
Population dynamics of enteric Salmonella in response to antimicrobial use in beef feedlot cattle.Sci Rep. 2017 Oct 30;7(1):14310. doi: 10.1038/s41598-017-14751-9. Sci Rep. 2017. PMID: 29085049 Free PMC article. Clinical Trial.
-
Multidrug-resistant enterobacteria in newborn dairy calves in Germany.PLoS One. 2021 Mar 12;16(3):e0248291. doi: 10.1371/journal.pone.0248291. eCollection 2021. PLoS One. 2021. PMID: 33711073 Free PMC article.
-
Impact of Parenteral Ceftiofur on Developmental Dynamics of Early Life Fecal Microbiota and Antibiotic Resistome in Neonatal Lambs.Antibiotics (Basel). 2025 Apr 25;14(5):434. doi: 10.3390/antibiotics14050434. Antibiotics (Basel). 2025. PMID: 40426501 Free PMC article.
-
Excreted Antibiotics May Be Key to Emergence of Increasingly Efficient Antibiotic Resistance in Food Animal Production.Appl Environ Microbiol. 2022 Aug 9;88(15):e0079122. doi: 10.1128/aem.00791-22. Epub 2022 Jul 14. Appl Environ Microbiol. 2022. PMID: 35867586 Free PMC article.
References
-
- WHO . WHO global strategy for containment of antimicrobial resistance. 2001.
-
- European Commission. Communication from the commission to the European parliament and council. Action plan against the rising threats from antimicrobial resistance. http://ec.europa.eu/dgs/health_consumer/docs/communication_amr_2011_748_....
-
- Council of the European Union. Council conclusions on the impact of antimicrobial resistance in the human health sector and in theveterinary sector—a ‘One Health’ perspective: http://www.consilium.europa.eu/uedocs/cms_data/docs/pressdata/en/lsa/131....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

