A phase 2 study of idelalisib plus rituximab in treatment-naïve older patients with chronic lymphocytic leukemia
- PMID: 26472751
- PMCID: PMC4732760
- DOI: 10.1182/blood-2015-03-630947
A phase 2 study of idelalisib plus rituximab in treatment-naïve older patients with chronic lymphocytic leukemia
Abstract
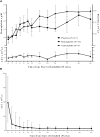
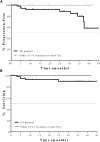
Idelalisib is a first-in-class oral inhibitor of PI3Kδ that has shown substantial activity in patients with relapsed/refractory chronic lymphocytic leukemia (CLL). To evaluate idelalisib as initial therapy, 64 treatment-naïve older patients with CLL or small lymphocytic leukemia (median age, 71 years; range, 65-90) were treated with rituximab 375 mg/m(2) weekly ×8 and idelalisib 150 mg twice daily continuously for 48 weeks. Patients completing 48 weeks without progression could continue to receive idelalisib on an extension study. The median time on treatment was 22.4 months (range, 0.8-45.8+). The overall response rate (ORR) was 97%, including 19% complete responses. The ORR was 100% in patients with del(17p)/TP53 mutations and 97% in those with unmutated IGHV. Progression-free survival was 83% at 36 months. The most frequent (>30%) adverse events (any grade) were diarrhea (including colitis) (64%), rash (58%), pyrexia (42%), nausea (38%), chills (36%), cough (33%), and fatigue (31%). Elevated alanine transaminase/aspartate transaminase was seen in 67% of patients (23% grade ≥3). The combination of idelalisib and rituximab was highly active, resulting in durable disease control in treatment-naïve older patients with CLL. These results support the further development of idelalisib as initial treatment of CLL. This study is registered at ClinicalTrials.gov as #NCT01203930.
© 2015 by The American Society of Hematology.
Figures
Comment in
-
Idelalisib has CLL on the run!Blood. 2015 Dec 17;126(25):2656-7. doi: 10.1182/blood-2015-10-676890. Blood. 2015. PMID: 26679541 No abstract available.
References
-
- Dighiero G, Hamblin TJ. Chronic lymphocytic leukaemia. Lancet. 2008;371(9617):1017–1029. - PubMed
-
- DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64(4):252–271. - PubMed
-
- Eichhorst B, Goede V, Hallek M. Treatment of elderly patients with chronic lymphocytic leukemia. Leuk Lymphoma. 2009;50(2):171–178. - PubMed
-
- Redaelli A, Laskin BL, Stephens JM, Botteman MF, Pashos CL. The clinical and epidemiological burden of chronic lymphocytic leukaemia. Eur J Cancer Care (Engl) 2004;13(3):279–287. - PubMed
-
- Keating MJ, O’Brien S, Kontoyiannis D, et al. Results of first salvage therapy for patients refractory to a fludarabine regimen in chronic lymphocytic leukemia. Leuk Lymphoma. 2002;43(9):1755–1762. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

