Randomized phase 2 study of obinutuzumab monotherapy in symptomatic, previously untreated chronic lymphocytic leukemia
- PMID: 26472752
- PMCID: PMC4705612
- DOI: 10.1182/blood-2015-03-634394
Randomized phase 2 study of obinutuzumab monotherapy in symptomatic, previously untreated chronic lymphocytic leukemia
Abstract
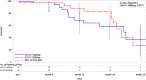
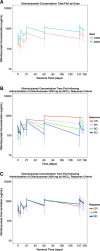
Obinutuzumab is a glycoengineered, type 2 anti-CD20 humanized antibody with single-agent activity in relapsed chronic lymphocytic leukemia (CLL). With other CD20 antibodies, a dose-response relationship has been shown. We therefore performed a randomized phase 2 study in symptomatic, untreated CLL patients to evaluate if an obinutuzumab dose response exists. Obinutuzumab was given at a dose of 1000 mg (100 mg IV day 1, 900 mg day 2, 1000 mg day 8 and day 15 of cycle 1; 1000 mg day 1 of cycles 2-8) or 2000 mg (100 mg IV day 1, 900 mg day 2, 1000 mg day 3, 2000 mg day 8 and day 15 of cycle 1; 2000 mg day 1 of cycles 2-8). The primary end point was overall response rate (ORR). Eighty patients were enrolled with similar demographics: median age 67 years, 41% high-risk Rai disease, and 10% del(17p)(13.1). ORR (67% vs 49%, P = .08) and complete response (CR) or CR with incomplete cytopenia response (20% vs 5%) favored 2000 mg obinutuzumab. Overall, therapy was well tolerated, and infusion events were manageable. This study demonstrates significant efficacy of obinutuzumab monotherapy, for 1000 mg as well as for 2000 mg, in untreated CLL patients with acceptable toxicity. Although exploratory, a dose-response relationship may exist, but its relevance to improving progression-free survival is uncertain and will require further follow-up. This trial was registered at www.clinicaltrials.gov as #NCT01414205.
© 2016 by The American Society of Hematology.
Figures

Comment in
-
Obinutuzumab: the more the merrier?Blood. 2016 Jan 7;127(1):6-8. doi: 10.1182/blood-2015-10-676882. Blood. 2016. PMID: 26744434 Free PMC article.
References
-
- Rai KR, Peterson BL, Appelbaum FR, et al. Fludarabine compared with chlorambucil as primary therapy for chronic lymphocytic leukemia. N Engl J Med. 2000;343(24):1750–1757. - PubMed
-
- Eichhorst BF, Busch R, Stilgenbauer S, et al. German CLL Study Group (GCLLSG) First-line therapy with fludarabine compared with chlorambucil does not result in a major benefit for elderly patients with advanced chronic lymphocytic leukemia. Blood. 2009;114(16):3382–3391. - PubMed
-
- Catovsky D, Richards S, Matutes E, et al. UK National Cancer Research Institute (NCRI) Haematological Oncology Clinical Studies Group; NCRI Chronic Lymphocytic Leukaemia Working Group. Assessment of fludarabine plus cyclophosphamide for patients with chronic lymphocytic leukaemia (the LRF CLL4 Trial): a randomised controlled trial. Lancet. 2007;370(9583):230–239. - PubMed
-
- Flinn IW, Neuberg DS, Grever MR, et al. Phase III trial of fludarabine plus cyclophosphamide compared with fludarabine for patients with previously untreated chronic lymphocytic leukemia: US Intergroup Trial E2997. J Clin Oncol. 2007;25(7):793–798. - PubMed
-
- Eichhorst BF, Busch R, Hopfinger G, et al. German CLL Study Group. Fludarabine plus cyclophosphamide versus fludarabine alone in first-line therapy of younger patients with chronic lymphocytic leukemia. Blood. 2006;107(3):885–891. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

