Multicenter Safety and Immunogenicity Trial of an Attenuated Measles Vaccine for NHP
- PMID: 26473350
- PMCID: PMC4617337
Multicenter Safety and Immunogenicity Trial of an Attenuated Measles Vaccine for NHP
Abstract
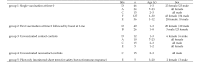
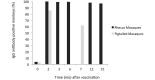
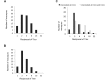
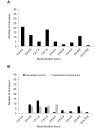
Measles is a highly contagious viral disease in NHP. The infection can range from asymptomatic to rapidly fatal, resulting in significant morbidity and mortality in captive populations. In addition to appropriate quarantine practices, restricted access, the immunization of all personnel in contact with NHP, and the wearing of protective clothing including face masks, measles immunization further reduces the infection risk. Commercially available measles vaccines are effective for use in NHP, but interruptions in their availability have prevented the implementation of ongoing, consistent vaccination programs. This need for a readily available vaccine led us to perform a broad, multicenter safety and immunogenicity study of another candidate vaccine, MVac (Serum Institute of India), a monovalent measles vaccine derived from live Edmonston-Zagreb strain virus that had been attenuated after 22 passages on human diploid cells.
Figures
Similar articles
-
Vaccine-induced measles virus-specific T cells do not prevent infection or disease but facilitate subsequent clearance of viral RNA.mBio. 2014 Apr 15;5(2):e01047. doi: 10.1128/mBio.01047-14. mBio. 2014. PMID: 24736226 Free PMC article.
-
Measles Vaccine.Viral Immunol. 2018 Mar;31(2):86-95. doi: 10.1089/vim.2017.0143. Epub 2017 Dec 19. Viral Immunol. 2018. PMID: 29256824 Free PMC article. Review.
-
Measles in the cynomolgus monkey (Macaca fascicularis).Vet Rec. 1989 Feb 25;124(8):184-6. doi: 10.1136/vr.124.8.184. Vet Rec. 1989. PMID: 2929098
-
A clinical study to assess the safety and immunogenicity of attenuated measles vaccine administered intranasally to healthy adults.Hum Vaccin. 2007 Mar-Apr;3(2):54-8. doi: 10.4161/hv.3.2.3877. Epub 2007 Mar 10. Hum Vaccin. 2007. PMID: 17312403 Clinical Trial.
-
Measles: old vaccines, new vaccines.Curr Top Microbiol Immunol. 2009;330:191-212. doi: 10.1007/978-3-540-70617-5_10. Curr Top Microbiol Immunol. 2009. PMID: 19203111 Review.
Cited by
-
Humanized Mice for Live-Attenuated Vaccine Research: From Unmet Potential to New Promises.Vaccines (Basel). 2020 Jan 21;8(1):36. doi: 10.3390/vaccines8010036. Vaccines (Basel). 2020. PMID: 31973073 Free PMC article. Review.
-
Modified Dose Efficacy Trial of a Canine Distemper-Measles Vaccine for Use in Rhesus Macaques (Macaca mulatta).J Am Assoc Lab Anim Sci. 2019 May 1;58(3):397-405. doi: 10.30802/AALAS-JAALAS-18-000091. Epub 2019 Mar 28. J Am Assoc Lab Anim Sci. 2019. PMID: 30922419 Free PMC article.
References
-
- Abee CR, Mansfield K, Tardif SD, Morris T. 2012. Nonhuman primates in biomedical research: biology and management, 2nd ed San Francisco (CA): Academic Press.
-
- Acha PN, Szyfres B. 2003. Zoonoses and communicable diseases common to man and animals: parasitic zoonoses, 3rd ed Washington (DC): Pan American Health Organization.
-
- Animal Welfare Act as Amended. 2013. 7 USC §2131–2159.
-
- Animal Welfare Regulations. 2013. 9 CFR § 3.129.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
