Peripheral Airway Smooth Muscle, but Not the Trachealis, Is Hypercontractile in an Equine Model of Asthma
- PMID: 26473389
- PMCID: PMC4942195
- DOI: 10.1165/rcmb.2015-0180OC
Peripheral Airway Smooth Muscle, but Not the Trachealis, Is Hypercontractile in an Equine Model of Asthma
Abstract
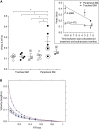
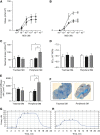
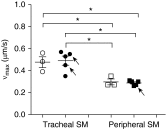
Heaves is a naturally occurring equine disease that shares many similarities with human asthma, including reversible antigen-induced bronchoconstriction, airway inflammation, and remodeling. The purpose of this study was to determine whether the trachealis muscle is mechanically representative of the peripheral airway smooth muscle (ASM) in an equine model of asthma. Tracheal and peripheral ASM of heaves-affected horses under exacerbation, or under clinical remission of the disease, and control horses were dissected and freed of epithelium to measure unloaded shortening velocity (Vmax), stress (force/cross-sectional area), methacholine effective concentration at which 50% of the maximum response is obtained, and stiffness. Myofibrillar Mg(2+)-ATPase activity, actomyosin in vitro motility, and contractile protein expression were also measured. Horses with heaves had significantly greater Vmax and Mg(2+)-ATPase activity in peripheral airway but not in tracheal smooth muscle. In addition, a significant correlation was found between Vmax and the time elapsed since the end of the corticosteroid treatment for the peripheral airways in horses with heaves. Maximal stress and stiffness were greater in the peripheral airways of the horses under remission compared with controls and the horses under exacerbation, potentially due to remodeling. Actomyosin in vitro motility was not different between controls and horses with heaves. These data demonstrate that peripheral ASM is mechanically and biochemically altered in heaves, whereas the trachealis behaves as in control horses. It is therefore conceivable that the trachealis muscle may not be representative of the peripheral ASM in human asthma either, but this will require further investigation.
Keywords: airway hyperresponsiveness; airway smooth muscle; asthma; smooth muscle mechanics.
Figures


References
-
- Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald M, Gibson P, Ohta K, O’Byrne P, Pedersen SE, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008;31:143–178. - PubMed
-
- Antonissen LA, Mitchell RW, Kroeger EA, Kepron W, Tse KS, Stephens NL. Mechanical alterations of airway smooth muscle in a canine asthmatic model. J Appl Physiol. 1979;46:681–687. - PubMed
-
- Jiang H, Rao K, Halayko AJ, Kepron W, Stephens NL. Bronchial smooth muscle mechanics of a canine model of allergic airway hyperresponsiveness. J Appl Physiol (1985) 1992;72:39–45. - PubMed
-
- Fan T, Yang M, Halayko A, Mohapatra SS, Stephens NL. Airway responsiveness in two inbred strains of mouse disparate in IgE and IL-4 production. Am J Respir Cell Mol Biol. 1997;17:156–163. - PubMed
-
- Duguet A, Biyah K, Minshall E, Gomes R, Wang CG, Taoudi-Benchekroun M, Bates JH, Eidelman DH. Bronchial responsiveness among inbred mouse strains: role of airway smooth-muscle shortening velocity. Am J Respir Crit Care Med. 2000;161:839–848. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

