Peripheral Vγ9Vδ2 T Cells Are a Novel Reservoir of Latent HIV Infection
- PMID: 26473478
- PMCID: PMC4608739
- DOI: 10.1371/journal.ppat.1005201
Peripheral Vγ9Vδ2 T Cells Are a Novel Reservoir of Latent HIV Infection
Abstract
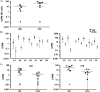
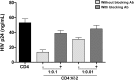
Eradication of HIV infection will require the identification of all cellular reservoirs that harbor latent infection. Despite low or lack of CD4 receptor expression on Vδ2 T cells, infection of these cells has previously been reported. We found that upregulation of the CD4 receptor may render primary Vδ2 cells target for HIV infection in vitro and we propose that HIV-induced immune activation may allow infection of γδ T cells in vivo. We assessed the presence of latent HIV infection by measurements of DNA and outgrowth assays within Vδ2 cells in 18 aviremic patients on long-standing antiretroviral therapy. In 14 patients we recovered latent but replication-competent HIV from highly purified Vδ2 cells demonstrating that peripheral Vδ2 T cells are a previously unrecognized reservoir in which latent HIV infection is unexpectedly frequent.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Chun TW, Carruth L, Finzi D, Shen X, DiGiuseppe JA, Taylor H, et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature 1997; 387: 183–188. - PubMed
-
- Finzi D, Blankson J, Siliciano JD, Margolisk JD, Chadwick K, Pierson T, et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med. 1999; 5: 512–517. - PubMed
-
- Wong J, Hezareh M, Günthard HF, Havlir DV, Ignacio CC, Richman DD. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia Science 1997; 278: 1291–1294. - PubMed
-
- Siliciano JD, Kajdas J, Finzi D, Quinn TC, Margolick JB, Kovacs C, et al. Longterm follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med. 2003; 9: 727–728. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

