Development of Non-Viral, Trophoblast-Specific Gene Delivery for Placental Therapy
- PMID: 26473479
- PMCID: PMC4608830
- DOI: 10.1371/journal.pone.0140879
Development of Non-Viral, Trophoblast-Specific Gene Delivery for Placental Therapy
Abstract
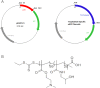
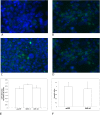
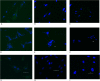
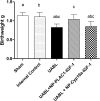
Low birth weight is associated with both short term problems and the fetal programming of adult onset diseases, including an increased risk of obesity, diabetes and cardiovascular disease. Placental insufficiency leading to intrauterine growth restriction (IUGR) contributes to the prevalence of diseases with developmental origins. Currently there are no therapies for IUGR or placental insufficiency. To address this and move towards development of an in utero therapy, we employ a nanostructure delivery system complexed with the IGF-1 gene to treat the placenta. IGF-1 is a growth factor critical to achieving appropriate placental and fetal growth. Delivery of genes to a model of human trophoblast and mouse placenta was achieved using a diblock copolymer (pHPMA-b-pDMAEMA) complexed to hIGF-1 plasmid DNA under the control of trophoblast-specific promoters (Cyp19a or PLAC1). Transfection efficiency of pEGFP-C1-containing nanocarriers in BeWo cells and non-trophoblast cells was visually assessed via fluorescence microscopy. In vivo transfection and functionality was assessed by direct placental-injection into a mouse model of IUGR. Complexes formed using pHPMA-b-pDMAEMA and CYP19a-923 or PLAC1-modified plasmids induce trophoblast-selective transgene expression in vitro, and placental injection of PLAC1-hIGF-1 produces measurable RNA expression and alleviates IUGR in our mouse model, consequently representing innovative building blocks towards human placental gene therapies.
Conflict of interest statement
Figures
References
-
- Ventolini G. Conditions associated with placental dysfunction. Minerva Ginecol. 2011;63(5):459–64. - PubMed
-
- McIntire DD, Bloom SL, Casey BM, Leveno KJ. Birth weight in relation to morbidity and mortality among newborn infants. N Engl J Med 1999;340:1234–1238. - PubMed
-
- Barker DJ. The developmental origins of adult disease. Eur J Epidemiol. 2003;18(8):733–6. - PubMed
-
- Fowden AL. The insulin-like growth factors and feto-placental growth. Placenta. 2003. 24(8–9):803–812. - PubMed
-
- Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell. 1993. 8;75(1):59–72 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

