Factor VIII Is Synthesized in Human Endothelial Cells, Packaged in Weibel-Palade Bodies and Secreted Bound to ULVWF Strings
- PMID: 26473492
- PMCID: PMC4608722
- DOI: 10.1371/journal.pone.0140740
Factor VIII Is Synthesized in Human Endothelial Cells, Packaged in Weibel-Palade Bodies and Secreted Bound to ULVWF Strings
Abstract
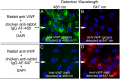
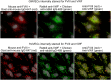
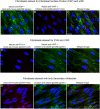
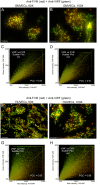
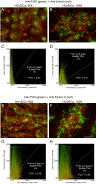
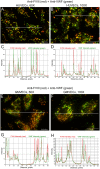
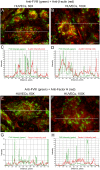
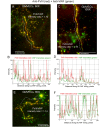
The cellular synthesis site and ensuing storage location for human factor VIII (FVIII), the coagulation protein deficient in hemophilia A, has been elusive. FVIII stability and half-life is dependent on non-covalent complex formation with von Willebrand factor (VWF) to avoid proteolysis and clearance. VWF is synthesized in megakaryocytes and endothelial cells, and is stored and secreted from platelet alpha granules and Weibel-Palade bodies of endothelial cells. In this paper we provide direct evidence for FVIII synthesis in 2 types of primary human endothelial cells: glomerular microvascular endothelial cells (GMVECs) and umbilical vein endothelial cells (HUVECs). Gene expression quantified by real time PCR revealed that levels of F8 and VWF are similar in GMVECs and HUVECs. Previous clinical studies have shown that stimulation of vasopressin V2 receptors causes parallel secretion of both proteins. In this study, we found that both endothelial cell types express AVPR2 (vasopressin V2 receptor gene) and that AVPR2 mRNA levels are 5-fold higher in GMVECs than HUVECs. FVIII and VWF proteins were detected by fluorescent microscopy in Weibel-Palade bodies within GMVECs and HUVECs using antibodies proven to be target specific. Visual presence of FVIII and VWF in Weibel-Palade bodies was confirmed by correlation measurements. The high extent of correlation was compared with negative correlation values obtained from FVIII detection with cytoplasmic proteins, β-actin and Factor H. FVIII activity was positive in GMVEC and HUVEC cell lysates. Stimulated GMVECs and HUVECs were found to secrete cell-anchored ultra-large VWF strings covered with bound FVIII.
Conflict of interest statement
Figures

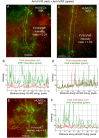
References
-
- Lenting PJ, Neels JG, van den Berg BM, Clijsters PP, Meijerman DW, Pannekoek H et al. The light chain of factor VIII comprises a binding site for low density lipoprotein receptor-related protein. J Biol Chem. 1999; 274: 23734–23739. - PubMed
-
- Lenting PJ, van Mourik JA, Mertens K. The life cycle of coagulation factor VIII in view of its structure and function. Blood. 1998; 92: 3983–3996. - PubMed
-
- Saenko EL, Yakhyaev AV, Mikhailenko I, Strickland DK, Sarafanov AG. Role of the low density lipoprotein-related protein receptor in mediation of factor VIII catabolism. J Biol Chem. 1999; 274: 37685–37692. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

