Factors Associated with Clinical Research Recruitment in a Pediatric Academic Medical Center--A Web-Based Survey
- PMID: 26473602
- PMCID: PMC4608599
- DOI: 10.1371/journal.pone.0140768
Factors Associated with Clinical Research Recruitment in a Pediatric Academic Medical Center--A Web-Based Survey
Abstract
Background: One of the most difficult aspects of conducting clinical research is the ability to successfully recruit participants. Pediatric clinical research presents unique recruitment challenges that relate to the need for parental consent on behalf of a minor, child assent, and school attendance. Yet, this has been less well studied. We conducted a survey of investigators performing human subjects research in a single large academic pediatric hospital to better understand characteristics of studies with successful recruitment.
Methods: We conducted a web-based survey from September 2011 to December 2011 of all principal investigators with an Institutional Review Board approved human subjects protocol at Boston Children's Hospital, a pediatric Academic Medical Center. The survey captured various characteristics of the protocols including study design, staffing, resources, and investigator experience and training as well as respondents' perceived barriers and facilitators to recruitment. We used chi square tests and Mantel-Haenszel test for linear trend to examine the relationship between selected predictor variables and the binary outcome of successful vs. unsuccessful recruitment and multivariable logistic regression analyses to examine the simultaneous influence of potential predictors on each outcome.
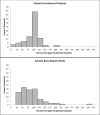
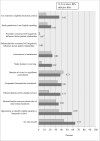
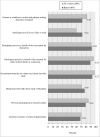
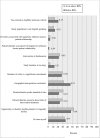
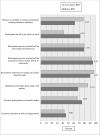
Results: Among the 349 eligible investigators, 52% responded to the survey, and 181 with valid data were included in the analyses. Two-thirds of the 87 protocols closed to enrollment reached 80% or more of their target enrollment, whereas, only one-third of the 94 protocols actively recruiting were meeting 80% of their target. Recruitment method appeared to be the only significant and independent factor associated with achieving 80% or more of target enrollment in closed to enrollment protocols. Closed to enrollment protocols that used recruitment in person were 4.55 times (95% CI 1.30 to 15.93; p = 0.02) more likely to achieve 80% or more of their target enrollment when compared to those that used other recruitment methods. Other potentially modifiable factors such as number of study visits, study duration and investigator experience were suggestive of being meaningfully related to recruitment.
Conclusion: Recruiting in person may promote reaching an acceptable target enrollment in pediatric as well as adult clinical research. Future research is needed on larger and more diverse samples to gain a better understanding of how the characteristics and qualifications of the individuals who conduct recruitment influence participant enrollment as well as how best to approach patient and families for their participation.
Conflict of interest statement
Figures
References
-
- Anderson DL. A Guide to Patient Recruitment—Today’s Best Practices and Proven Strategies. Boston: CenterWatch; 2002.
-
- Treweek S, Pitkethly M, Cook J, Kjeldstrøm M, Taskila T, Johansen M, et al. Strategies to improve recruitment to randomised controlled trials. Cochrane Database Syst Rev. 2010; 14;43. - PubMed
-
- Hunninghake DB, Darby CA, Probstfield JL. Recruitment experience in clinical trials: literature summary and annotated bibliography. Control Clin Trials. 1987; 8 (4 Suppl): 6S–30S. - PubMed
-
- Ross S, Grant A, Counsell C, Gillespie W, Russell I, Prescott R. Barriers to participation in randomised controlled trials: a systematic review. J Clin Epidemiol. 1999;52(12):1143–56. - PubMed
-
- Fayter D, McDaid C, Eastwood A. A systematic review highlights threats to validity in studies of barriers to cancer trial participation. J Clin Epidemiol. 2007; 60(10):990–1001. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

