Anti-Tumor Effects after Adoptive Transfer of IL-12 Transposon-Modified Murine Splenocytes in the OT-I-Melanoma Mouse Model
- PMID: 26473608
- PMCID: PMC4608718
- DOI: 10.1371/journal.pone.0140744
Anti-Tumor Effects after Adoptive Transfer of IL-12 Transposon-Modified Murine Splenocytes in the OT-I-Melanoma Mouse Model
Abstract
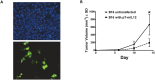
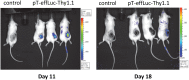
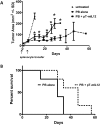
Adoptive transfer of gene modified T cells provides possible immunotherapy for patients with cancers refractory to other treatments. We have previously used the non-viral piggyBac transposon system to gene modify human T cells for potential immunotherapy. However, these previous studies utilized adoptive transfer of modified human T cells to target cancer xenografts in highly immunodeficient (NOD-SCID) mice that do not recapitulate an intact immune system. Currently, only viral vectors have shown efficacy in permanently gene-modifying mouse T cells for immunotherapy applications. Therefore, we sought to determine if piggyBac could effectively gene modify mouse T cells to target cancer cells in a mouse cancer model. We first demonstrated that we could gene modify cells to express murine interleukin-12 (p35/p40 mIL-12), a transgene with proven efficacy in melanoma immunotherapy. The OT-I melanoma mouse model provides a well-established T cell mediated immune response to ovalbumin (OVA) positive B16 melanoma cells. B16/OVA melanoma cells were implanted in wild type C57Bl6 mice. Mouse splenocytes were isolated from C57Bl6 OT-I mice and were gene modified using piggyBac to express luciferase. Adoptive transfer of luciferase-modified OT-I splenocytes demonstrated homing to B16/OVA melanoma tumors in vivo. We next gene-modified OT-I cells to express mIL-12. Adoptive transfer of mIL-12-modified mouse OT-I splenocytes delayed B16/OVA melanoma tumor growth in vivo compared to control OT-I splenocytes and improved mouse survival. Our results demonstrate that the piggyBac transposon system can be used to gene modify splenocytes and mouse T cells for evaluating adoptive immunotherapy strategies in immunocompetent mouse tumor models that may more directly mimic immunotherapy applications in humans.
Conflict of interest statement
Figures



Similar articles
-
Interleukin-21 restrains tumor growth and induces a substantial increase in the number of circulating tumor-specific T cells in a murine model of malignant melanoma.Cytokine. 2010 Jan;49(1):80-8. doi: 10.1016/j.cyto.2009.11.001. Epub 2009 Dec 3. Cytokine. 2010. PMID: 19962321
-
Therapeutic effects of adoptive splenocyte transfer following in situ AdIL-12 gene therapy in a mouse prostate cancer model.Cancer Gene Ther. 2006 Jan 1;13(1):91-8. doi: 10.1038/sj.cgt.7700872. Cancer Gene Ther. 2006. PMID: 16052232
-
Adoptive transfer of membrane-restricted IL-12-TCR T cells promotes antigen spreading and elimination of antigen-negative tumor variants.J Immunother Cancer. 2024 Nov 18;12(11):e009868. doi: 10.1136/jitc-2024-009868. J Immunother Cancer. 2024. PMID: 39557544 Free PMC article.
-
Adoptive immunotherapy of cancer with immune and activated lymphocytes: experimental and clinical studies.Ric Clin Lab. 1986 Jan-Mar;16(1):1-20. doi: 10.1007/BF02886719. Ric Clin Lab. 1986. PMID: 2874605 Review.
-
New perspectives for melanoma immunotherapy: role of IL-12.Curr Mol Med. 2009 May;9(4):459-69. doi: 10.2174/156652409788167140. Curr Mol Med. 2009. PMID: 19519403 Review.
Cited by
-
Antitumor Activities of Interleukin-12 in Melanoma.Cancers (Basel). 2022 Nov 14;14(22):5592. doi: 10.3390/cancers14225592. Cancers (Basel). 2022. PMID: 36428682 Free PMC article. Review.
-
Kidney-specific transposon-mediated gene transfer in vivo.Sci Rep. 2017 Mar 20;7:44904. doi: 10.1038/srep44904. Sci Rep. 2017. PMID: 28317878 Free PMC article.
-
Autologous iPSC-Based Vaccines Elicit Anti-tumor Responses In Vivo.Cell Stem Cell. 2018 Apr 5;22(4):501-513.e7. doi: 10.1016/j.stem.2018.01.016. Epub 2018 Feb 15. Cell Stem Cell. 2018. PMID: 29456158 Free PMC article.
-
Interleukin-12 Delivery Strategies and Advances in Tumor Immunotherapy.Curr Issues Mol Biol. 2024 Oct 16;46(10):11548-11579. doi: 10.3390/cimb46100686. Curr Issues Mol Biol. 2024. PMID: 39451566 Free PMC article. Review.
-
Genome Engineering of Human Urine-Derived Stem Cells to Express Lactoferrin and Deoxyribonuclease.Tissue Eng Part A. 2023 Jul;29(13-14):372-383. doi: 10.1089/ten.TEA.2023.0003. Epub 2023 Jun 2. Tissue Eng Part A. 2023. PMID: 37130035 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

