Injurious Loading of Articular Cartilage Compromises Chondrocyte Respiratory Function
- PMID: 26473613
- PMCID: PMC4767543
- DOI: 10.1002/art.39460
Injurious Loading of Articular Cartilage Compromises Chondrocyte Respiratory Function
Abstract
Objective: To determine whether repeatedly overloading healthy cartilage disrupts mitochondrial function in a manner similar to that associated with osteoarthritis (OA) pathogenesis.
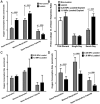
Methods: We exposed normal articular cartilage on bovine osteochondral explants to 1 day or 7 consecutive days of cyclic axial compression (0.25 MPa or 1.0 MPa at 0.5 Hz for 3 hours) and evaluated the effects on chondrocyte viability, ATP concentration, reactive oxygen species (ROS) production, indicators of oxidative stress, respiration, and mitochondrial membrane potential.
Results: Neither 0.25 MPa nor 1.0 MPa of cyclic compression caused extensive chondrocyte death, macroscopic tissue damage, or overt changes in stress-strain behavior. After 1 day of loading, differences in respiratory activities between the 0.25 MPa and 1.0 MPa groups were minimal; however, after 7 days of loading, respiratory activity and ATP levels were suppressed in the 1.0 MPa group relative to the 0.25 MPa group, an effect prevented by pretreatment with 10 mM N-acetylcysteine. These changes were accompanied by increased proton leakage and decreased mitochondrial membrane potential, as well as by increased ROS formation, as indicated by dihydroethidium staining and glutathione oxidation.
Conclusion: Repeated overloading leads to chondrocyte oxidant-dependent mitochondrial dysfunction. This mitochondrial dysfunction may contribute to destabilization of cartilage during various stages of OA in distinct ways by disrupting chondrocyte anabolic responses to mechanical stimuli.
© 2016, American College of Rheumatology.
Conflict of interest statement
Mitchell Coleman, James Martin, Marc Brouillette and Prem Ramakrishnan have no conflicts of interest or other disclosures.
Figures




References
-
- Buckwalter JA, Martin JA. Sports and osteoarthritis. Curr Opin Rheumatol. 2004;16:634–9. Review. - PubMed
-
- Egloff C, Hugle T, Valderrabano V. Biomechanics and pathomechanisms of osteoarthritis. Swiss Med Wkly. 2012;142:w13583. Review. - PubMed
-
- Baars DC, Rundell SA, Haut RC. Treatment with the non-ionic surfactant poloxamer P188 reduces DNA fragmentation in cells from bovine chondral explants exposed to injurious unconfined compression. Biomech Model Mechanobiol. 2006;5:133–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

