Phosphorylation and Internalization of Lysophosphatidic Acid Receptors LPA1, LPA2, and LPA3
- PMID: 26473723
- PMCID: PMC4608732
- DOI: 10.1371/journal.pone.0140583
Phosphorylation and Internalization of Lysophosphatidic Acid Receptors LPA1, LPA2, and LPA3
Abstract
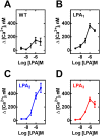
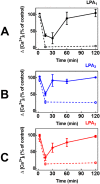
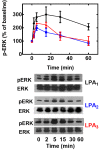
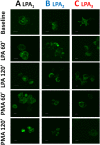
Results: The lysophosphatidic acid receptors LPA1, LPA2, and LPA3 were individually expressed in C9 cells and their signaling and regulation were studied. Agonist-activation increases intracellular calcium concentration in a concentration-dependent fashion. Phorbol myristate acetate markedly inhibited LPA1- and LPA3-mediated effect, whereas that mediated by LPA2 was only partially diminished; the actions of the phorbol ester were inhibited by bisindolylmaleimide I and by overnight incubation with the protein kinase C activator, which leads to down regulation of this protein kinase. Homologous desensitization was also observed for the three LPA receptors studied, with that of LPA2 receptors being consistently of lesser magnitude; neither inhibition nor down-regulation of protein kinase C exerted any effect on homologous desensitization. Activation of LPA1-3 receptors induced ERK 1/2 phosphorylation; this effect was markedly attenuated by inhibition of epidermal growth factor receptor tyrosine kinase activity, suggesting growth factor receptor transactivation in this effect. Lysophosphatidic acid and phorbol myristate acetate were able to induce LPA1-3 phosphorylation, in time- and concentration-dependent fashions. It was also clearly observed that agonists and protein kinase C activation induced internalization of these receptors. Phosphorylation of the LPA2 subtype required larger concentrations of these agents and its internalization was less intense than that of the other subtypes.
Conclusion: Our data show that these three LPA receptors are phosphoproteins whose phosphorylation state is modulated by agonist-stimulation and protein kinase C-activation and that differences in regulation and cellular localization exist, among the subtypes.
Conflict of interest statement
Figures












References
-
- Hernández-Méndez A, Alcántara-Hernández R., García-Sáinz J. A. Lysophosphatidic Acid LPA 1–3 receptors: signaling, regulation and in silico analysis of their putative phosphorylation sites. Recept Clin Invest. 2014;1:236–52. 10.14800/rci.193 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

