A Follicle Rupture Assay Reveals an Essential Role for Follicular Adrenergic Signaling in Drosophila Ovulation
- PMID: 26473732
- PMCID: PMC4608792
- DOI: 10.1371/journal.pgen.1005604
A Follicle Rupture Assay Reveals an Essential Role for Follicular Adrenergic Signaling in Drosophila Ovulation
Abstract
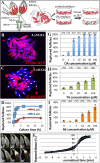
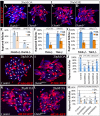
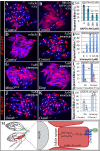
Ovulation is essential for the propagation of the species and involves a proteolytic degradation of the follicle wall for the release of the fertilizable oocyte. However, the precise mechanisms for regulating these proteolytic events are largely unknown. Work from our lab and others have shown that there are several parallels between Drosophila and mammalian ovulation at both the cellular and molecular levels. During ovulation in Drosophila, posterior follicle cells surrounding a mature oocyte are selectively degraded and the residual follicle cells remain in the ovary to form a corpus luteum after follicle rupture. Like in mammals, this rupturing process also depends on matrix metalloproteinase 2 (Mmp2) activity localized at the posterior end of mature follicles, where oocytes exit. In the present study, we show that Mmp2 activity is regulated by the octopaminergic signaling in mature follicle cells. Exogenous octopamine (OA; equivalent to norepinephrine, NE) is sufficient to induce follicle rupture when isolated mature follicles are cultured ex vivo, in the absence of the oviduct or ovarian muscle sheath. Knocking down the alpha-like adrenergic receptor Oamb (Octoampine receptor in mushroom bodies) in mature follicle cells prevents OA-induced follicle rupture ex vivo and ovulation in vivo. We also show that follicular OA-Oamb signaling induces Mmp2 enzymatic activation but not Mmp2 protein expression, likely via intracellular Ca2+ as the second messenger. Our work develops a novel ex vivo follicle rupture assay and demonstrates the role for follicular adrenergic signaling in Mmp2 activation and ovulation in Drosophila, which is likely conserved in other species.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Actomyosin contraction in the follicular epithelium provides the major mechanical force for follicle rupture during Drosophila ovulation.Proc Natl Acad Sci U S A. 2024 Sep 24;121(39):e2407083121. doi: 10.1073/pnas.2407083121. Epub 2024 Sep 18. Proc Natl Acad Sci U S A. 2024. PMID: 39292751 Free PMC article.
-
NADPH oxidase-generated reactive oxygen species in mature follicles are essential for Drosophila ovulation.Proc Natl Acad Sci U S A. 2018 Jul 24;115(30):7765-7770. doi: 10.1073/pnas.1800115115. Epub 2018 Jul 9. Proc Natl Acad Sci U S A. 2018. PMID: 29987037 Free PMC article.
-
Matrix metalloproteinase 2 is required for ovulation and corpus luteum formation in Drosophila.PLoS Genet. 2015 Feb 19;11(2):e1004989. doi: 10.1371/journal.pgen.1004989. eCollection 2015 Feb. PLoS Genet. 2015. PMID: 25695427 Free PMC article.
-
Ovarian follicular and luteal physiology.Int Rev Physiol. 1980;22:117-201. Int Rev Physiol. 1980. PMID: 6248477 Review.
-
An attempt to search for the common cellular mechanism of ovulation across all metazoans.Reproduction. 2025 Jan 2;169(1):e240184. doi: 10.1530/REP-24-0184. Print 2025 Jan 1. Reproduction. 2025. PMID: 39441770 Review.
Cited by
-
Ex vivo Follicle Rupture and in situ Zymography in Drosophila.Bio Protoc. 2018 May 20;8(10):e2846. doi: 10.21769/BioProtoc.2846. Bio Protoc. 2018. PMID: 29911127 Free PMC article.
-
Precise CRISPR-Cas9-mediated mutation of a membrane trafficking domain in the Drosophila vesicular monoamine transporter gene.Curr Res Physiol. 2023 Jun 20;6:100101. doi: 10.1016/j.crphys.2023.100101. eCollection 2023. Curr Res Physiol. 2023. PMID: 37409154 Free PMC article.
-
The zinc-finger transcription factor Hindsight regulates ovulation competency of Drosophila follicles.Elife. 2017 Dec 19;6:e29887. doi: 10.7554/eLife.29887. Elife. 2017. PMID: 29256860 Free PMC article.
-
Actomyosin contraction in the follicular epithelium provides the major mechanical force for follicle rupture during Drosophila ovulation.Proc Natl Acad Sci U S A. 2024 Sep 24;121(39):e2407083121. doi: 10.1073/pnas.2407083121. Epub 2024 Sep 18. Proc Natl Acad Sci U S A. 2024. PMID: 39292751 Free PMC article.
-
Quantitative analyses of EGFR localization and trafficking dynamics in the follicular epithelium.Development. 2020 Aug 14;147(15):dev183210. doi: 10.1242/dev.183210. Development. 2020. PMID: 32680934 Free PMC article.
References
-
- Cisint S, Crespo CA, Medina MF, Iruzubieta Villagra L, Fernández SN, Ramos I. Innervation of amphibian reproductive system. Histological and ultrastructural studies. Auton Neurosci Basic Clin. 2014;185: 51–58. - PubMed
-
- Gerendai I, Banczerowski P, Halász B. Functional significance of the innervation of the gonads. Endocrine. 2005;28: 309–318. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

