Modeling Influenza Virus Infection: A Roadmap for Influenza Research
- PMID: 26473911
- PMCID: PMC4632383
- DOI: 10.3390/v7102875
Modeling Influenza Virus Infection: A Roadmap for Influenza Research
Abstract
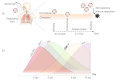
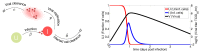
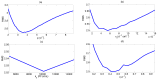
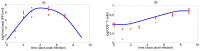
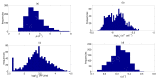
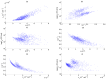
Influenza A virus (IAV) infection represents a global threat causing seasonal outbreaks and pandemics. Additionally, secondary bacterial infections, caused mainly by Streptococcus pneumoniae, are one of the main complications and responsible for the enhanced morbidity and mortality associated with IAV infections. In spite of the significant advances in our knowledge of IAV infections, holistic comprehension of the interplay between IAV and the host immune response (IR) remains largely fragmented. During the last decade, mathematical modeling has been instrumental to explain and quantify IAV dynamics. In this paper, we review not only the state of the art of mathematical models of IAV infection but also the methodologies exploited for parameter estimation. We focus on the adaptive IR control of IAV infection and the possible mechanisms that could promote a secondary bacterial coinfection. To exemplify IAV dynamics and identifiability issues, a mathematical model to explain the interactions between adaptive IR and IAV infection is considered. Furthermore, in this paper we propose a roadmap for future influenza research. The development of a mathematical modeling framework with a secondary bacterial coinfection, immunosenescence, host genetic factors and responsiveness to vaccination will be pivotal to advance IAV infection understanding and treatment optimization.
Keywords: aging; coinfection; host genetic factors; influenza; mathematical models; parameters estimation; vaccinology.
Figures
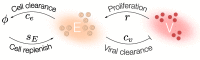

Similar articles
-
Memory Th17 cell-mediated protection against lethal secondary pneumococcal pneumonia following influenza infection.mBio. 2023 Aug 31;14(4):e0051923. doi: 10.1128/mbio.00519-23. Epub 2023 May 24. mBio. 2023. PMID: 37222516 Free PMC article.
-
Interactions between Streptococcus pneumoniae and influenza virus: a mutually beneficial relationship?Future Microbiol. 2012 May;7(5):609-24. doi: 10.2217/fmb.12.29. Future Microbiol. 2012. PMID: 22568716 Review.
-
Influenza viral neuraminidase primes bacterial coinfection through TGF-β-mediated expression of host cell receptors.Proc Natl Acad Sci U S A. 2015 Jan 6;112(1):238-43. doi: 10.1073/pnas.1414422112. Epub 2014 Dec 22. Proc Natl Acad Sci U S A. 2015. PMID: 25535343 Free PMC article.
-
GP96 Drives Exacerbation of Secondary Bacterial Pneumonia following Influenza A Virus Infection.mBio. 2021 Jun 29;12(3):e0326920. doi: 10.1128/mBio.03269-20. Epub 2021 Jun 1. mBio. 2021. PMID: 34061598 Free PMC article.
-
Secondary streptococcal infection following influenza.Microbiol Immunol. 2022 Jun;66(6):253-263. doi: 10.1111/1348-0421.12965. Epub 2022 May 26. Microbiol Immunol. 2022. PMID: 35088451 Review.
Cited by
-
Mathematical Modeling of the Lethal Synergism of Coinfecting Pathogens in Respiratory Viral Infections: A Review.Microorganisms. 2023 Dec 13;11(12):2974. doi: 10.3390/microorganisms11122974. Microorganisms. 2023. PMID: 38138118 Free PMC article. Review.
-
Oseltamivir PK/PD Modeling and Simulation to Evaluate Treatment Strategies against Influenza-Pneumococcus Coinfection.Front Cell Infect Microbiol. 2016 Jun 14;6:60. doi: 10.3389/fcimb.2016.00060. eCollection 2016. Front Cell Infect Microbiol. 2016. PMID: 27379214 Free PMC article.
-
Analysis of multi-strain infection of vaccinated and recovered population through epidemic model: Application to COVID-19.PLoS One. 2022 Jul 29;17(7):e0271446. doi: 10.1371/journal.pone.0271446. eCollection 2022. PLoS One. 2022. PMID: 35905113 Free PMC article.
-
Structure and Hierarchy of Influenza Virus Models Revealed by Reaction Network Analysis.Viruses. 2019 May 16;11(5):449. doi: 10.3390/v11050449. Viruses. 2019. PMID: 31100972 Free PMC article.
-
Towards a Mathematical Model for the Viral Progression in the Pharynx.Healthcare (Basel). 2021 Dec 20;9(12):1766. doi: 10.3390/healthcare9121766. Healthcare (Basel). 2021. PMID: 34946492 Free PMC article.
References
-
- World Health Organization (WHO) Influenza (Seasonal) Factsheet N 211. WHO; Geneva, Switzerland: 2009.
-
- World Health Organization (WHO) Influenza (Seasonal): Fact Sheet N∘211. WHO; Geneva, Switzerland: 2014.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

