Knockout Serum Replacement Promotes Cell Survival by Preventing BIM from Inducing Mitochondrial Cytochrome C Release
- PMID: 26473951
- PMCID: PMC4608728
- DOI: 10.1371/journal.pone.0140585
Knockout Serum Replacement Promotes Cell Survival by Preventing BIM from Inducing Mitochondrial Cytochrome C Release
Abstract
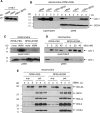
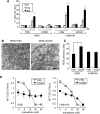
Knockout serum replacement (KOSR) is a nutrient supplement commonly used to replace serum for culturing stem cells. We show here that KOSR has pro-survival activity in chronic myelogenous leukemia (CML) cells transformed by the BCR-ABL oncogene. Inhibitors of BCR-ABL tyrosine kinase kill CML cells by stimulating pro-apoptotic BIM and inhibiting anti-apoptotic BCL2, BCLxL and MCL1. We found that KOSR protects CML cells from killing by BCR-ABL inhibitors--imatinib, dasatinib and nilotinib. The protective effect of KOSR is reversible and not due to the selective outgrowth of drug-resistant clones. In KOSR-protected CML cells, imatinib still inhibited the BCR-ABL tyrosine kinase, reduced the phosphorylation of STAT, ERK and AKT, down-regulated BCL2, BCLxL, MCL1 and up-regulated BIM. However, these pro-apoptotic alterations failed to cause cytochrome c release from the mitochondria. With mitochondria isolated from KOSR-cultured CML cells, we showed that addition of recombinant BIM protein also failed to cause cytochrome c release. Besides the kinase inhibitors, KOSR could protect cells from menadione, an inducer of oxidative stress, but it did not protect cells from DNA damaging agents. Switching from serum to KOSR caused a transient increase in reactive oxygen species and AKT phosphorylation in CML cells that were protected by KOSR but not in those that were not protected by this nutrient supplement. Treatment of KOSR-cultured cells with the PH-domain inhibitor MK2206 blocked AKT phosphorylation, abrogated the formation of BIM-resistant mitochondria and stimulated cell death. These results show that KOSR has cell-context dependent pro-survival activity that is linked to AKT activation and the inhibition of BIM-induced cytochrome c release from the mitochondria.
Conflict of interest statement
Figures







References
-
- Zabriskie MS, Eide CA, Tantravahi SK, Vellore NA, Estrada J, Nicolini FE, et al. BCR-ABL1 compound mutations combining key kinase domain positions confer clinical resistance to ponatinib in Ph chromosome-positive leukemia. Cancer Cell. 2014;26(3):428–42. Epub 2014/08/19. 10.1016/j.ccr.2014.07.006 - DOI - PMC - PubMed
-
- Asmussen J, Lasater EA, Tajon C, Oses-Prieto J, Jun YW, Taylor BS, et al. MEK-dependent negative feedback underlies BCR-ABL-mediated oncogene addiction. Cancer Discov. 2014;4(2):200–15. Epub 2013/12/24. 10.1158/2159-8290.CD-13-0235 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

