Identifying the Role of E2 Domains on Alphavirus Neutralization and Protective Immune Responses
- PMID: 26473963
- PMCID: PMC4608762
- DOI: 10.1371/journal.pntd.0004163
Identifying the Role of E2 Domains on Alphavirus Neutralization and Protective Immune Responses
Abstract
Background: Chikungunya virus (CHIKV) and other alphaviruses are the etiologic agents of numerous diseases in both humans and animals. Despite this, the viral mediators of protective immunity against alphaviruses are poorly understood, highlighted by the lack of a licensed human vaccine for any member of this virus genus. The alphavirus E2, the receptor-binding envelope protein, is considered to be the predominant target of the protective host immune response. Although envelope protein domains have been studied for vaccine and neutralization in flaviviruses, their role in alphaviruses is less characterized. Here, we describe the role of the alphavirus E2 domains in neutralization and protection through the use of chimeric viruses.
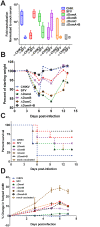
Methodology/principal findings: Four chimeric viruses were constructed in which individual E2 domains of CHIKV were replaced with the corresponding domain from Semliki Forest virus (SFV) (ΔDomA/ΔDomB/ΔDomC/ ΔDomA+B). Vaccination studies in mice (both live and inactivated virus) revealed that domain B was the primary determinant of neutralization. Neutralization studies with CHIKV immune serum from humans were consistent with mouse studies, as ΔDomB was poorly neutralized.
Conclusions/significance: Using chimeric viruses, it was determined that the alphavirus E2 domain B was the critical target of neutralizing antibodies in both mice and humans. Therefore, chimeric viruses may have more relevance for vaccine discovery than peptide-based approaches, which only detect linear epitopes. This study provides new insight into the role of alphavirus E2 domains on neutralization determinants and may be useful for the design of novel therapeutic technologies.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- CDC. Where Has Chikungunya Virus Been Found? CDC.gov2015.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

