Physiological modulation of BiP activity by trans-protomer engagement of the interdomain linker
- PMID: 26473973
- PMCID: PMC4608358
- DOI: 10.7554/eLife.08961
Physiological modulation of BiP activity by trans-protomer engagement of the interdomain linker
Abstract
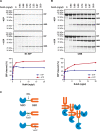
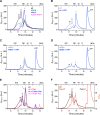
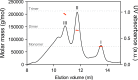
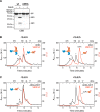
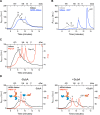
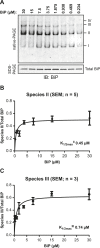
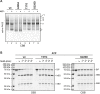
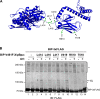
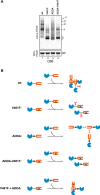
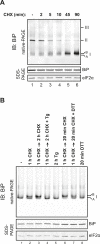
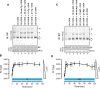
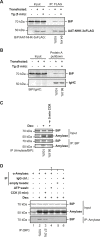
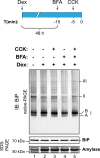
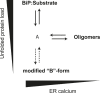
DnaK/Hsp70 chaperones form oligomers of poorly understood structure and functional significance. Site-specific proteolysis and crosslinking were used to probe the architecture of oligomers formed by the endoplasmic reticulum (ER) Hsp70, BiP. These were found to consist of adjacent protomers engaging the interdomain linker of one molecule in the substrate binding site of another, attenuating the chaperone function of oligomeric BiP. Native gel electrophoresis revealed a rapidly-modulated reciprocal relationship between the burden of unfolded proteins and BiP oligomers and slower equilibration between oligomers and inactive, covalently-modified BiP. Lumenal ER calcium depletion caused rapid oligomerization of mammalian BiP and a coincidental diminution in substrate binding, pointing to the relative inertness of the oligomers. Thus, equilibration between inactive oligomers and active monomeric BiP is poised to buffer fluctuations in ER unfolded protein load on a rapid timescale attainable neither by inter-conversion of active and covalently-modified BiP nor by the conventional unfolded protein response.
Keywords: BiP/GRP78; Hsp70; biochemistry; cell biology; endoplasmic reticulum; none; oligomerization.
Conflict of interest statement
DR: Reviewing editor,
The other authors declare that no competing interests exist.
Figures









Similar articles
-
Chaperone BiP controls ER stress sensor Ire1 through interactions with its oligomers.Life Sci Alliance. 2024 Aug 5;7(10):e202402702. doi: 10.26508/lsa.202402702. Print 2024 Oct. Life Sci Alliance. 2024. PMID: 39103227 Free PMC article.
-
AMPylation matches BiP activity to client protein load in the endoplasmic reticulum.Elife. 2015 Dec 17;4:e12621. doi: 10.7554/eLife.12621. Elife. 2015. PMID: 26673894 Free PMC article.
-
Calcium depletion challenges endoplasmic reticulum proteostasis by destabilising BiP-substrate complexes.Elife. 2020 Dec 9;9:e62601. doi: 10.7554/eLife.62601. Elife. 2020. PMID: 33295873 Free PMC article.
-
Early Events in the Endoplasmic Reticulum Unfolded Protein Response.Cold Spring Harb Perspect Biol. 2019 Apr 1;11(4):a033894. doi: 10.1101/cshperspect.a033894. Cold Spring Harb Perspect Biol. 2019. PMID: 30396883 Free PMC article. Review.
-
AMPylation and Endoplasmic Reticulum Protein Folding Homeostasis.Cold Spring Harb Perspect Biol. 2023 Mar 1;15(3):a041265. doi: 10.1101/cshperspect.a041265. Cold Spring Harb Perspect Biol. 2023. PMID: 36041787 Free PMC article. Review.
Cited by
-
Structural and functional analysis of the Hsp70/Hsp40 chaperone system.Protein Sci. 2020 Feb;29(2):378-390. doi: 10.1002/pro.3725. Epub 2019 Oct 7. Protein Sci. 2020. PMID: 31509306 Free PMC article. Review.
-
Production of an Active, Human Membrane Protein in Saccharomyces cerevisiae: Full-Length FICD.Int J Mol Sci. 2022 Feb 23;23(5):2458. doi: 10.3390/ijms23052458. Int J Mol Sci. 2022. PMID: 35269596 Free PMC article.
-
The protein kinase PERK/EIF2AK3 regulates proinsulin processing not via protein synthesis but by controlling endoplasmic reticulum chaperones.J Biol Chem. 2018 Apr 6;293(14):5134-5149. doi: 10.1074/jbc.M117.813790. Epub 2018 Feb 14. J Biol Chem. 2018. PMID: 29444822 Free PMC article.
-
Protein Aggregation in the ER: Calm behind the Storm.Cells. 2021 Nov 28;10(12):3337. doi: 10.3390/cells10123337. Cells. 2021. PMID: 34943844 Free PMC article. Review.
-
Enzymes Involved in AMPylation and deAMPylation.Chem Rev. 2018 Feb 14;118(3):1199-1215. doi: 10.1021/acs.chemrev.7b00145. Epub 2017 Aug 18. Chem Rev. 2018. PMID: 28819965 Free PMC article. Review.
References
-
- Aprile FA, Dhulesia A, Stengel F, Roodveldt C, Benesch JL, Tortora P, Robinson CV, Salvatella X, Dobson CM, Cremades N. Hsp70 oligomerization is mediated by an interaction between the interdomain linker and the substrate-binding domain. PLOS ONE. 2013;8:e67961. doi: 10.1371/journal.pone.0067961. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

